Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins
- PMID: 24289175
- PMCID: PMC3898251
- DOI: 10.1186/1471-2105-14-346
Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins
Abstract
Background: Disulfide engineering is an important biotechnological tool that has advanced a wide range of research. The introduction of novel disulfide bonds into proteins has been used extensively to improve protein stability, modify functional characteristics, and to assist in the study of protein dynamics. Successful use of this technology is greatly enhanced by software that can predict pairs of residues that will likely form a disulfide bond if mutated to cysteines.
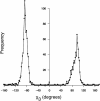
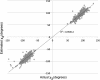
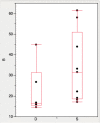
Results: We had previously developed and distributed software for this purpose: Disulfide by Design (DbD). The original DbD program has been widely used; however, it has a number of limitations including a Windows platform dependency. Here, we introduce Disulfide by Design 2.0 (DbD2), a web-based, platform-independent application that significantly extends functionality, visualization, and analysis capabilities beyond the original program. Among the enhancements to the software is the ability to analyze the B-factor of protein regions involved in predicted disulfide bonds. Importantly, this feature facilitates the identification of potential disulfides that are not only likely to form but are also expected to provide improved thermal stability to the protein.
Conclusions: DbD2 provides platform-independent access and significantly extends the original functionality of DbD. A web server hosting DbD2 is provided at http://cptweb.cpt.wayne.edu/DbD2/.
Figures

References
-
- Flory PJ. Theory of elastic mechanisms in fibrous proteins. J Am Chem Soc. 1956;14(20):5222–5235. doi: 10.1021/ja01601a025. - DOI
-
- Pace CN, Grimsley GR, Thomson JA, Barnett BJ. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem. 1988;14(24):11820–11825. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

