Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: a systematic review and meta-analysis
- PMID: 24289230
- PMCID: PMC4056781
- DOI: 10.1186/cc13134
Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: a systematic review and meta-analysis
Abstract
Introduction: The aim of this study was to determine whether using pharmacodynamic-based dosing of antimicrobials, such as extended/continuous infusions, in critically ill patients is associated with improved outcomes as compared with traditional dosing methods.
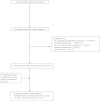
Methods: We searched Medline, HealthStar, EMBASE, Cochrane Clinical Trial Registry, and CINAHL from inception to September 2013 without language restrictions for studies comparing the use of extended/continuous infusions with traditional dosing. Two authors independently selected studies, extracted data on methodology and outcomes, and performed quality assessment. Meta-analyses were performed by using random-effects models.
Results: Of 1,319 citations, 13 randomized controlled trials (RCTs) (n = 782 patients) and 13 cohort studies (n = 2,117 patients) met the inclusion criteria. Compared with traditional non-pharmacodynamic-based dosing, RCTs of continuous/extended infusions significantly reduced clinical failure rates (relative risk (RR) 0.68; 95% confidence interval (CI) 0.49 to 0.94, P = 0.02) and intensive care unit length of stay (mean difference, -1.5; 95% CI, -2.8 to -0.2 days, P = 0.02), but not mortality (RR, 0.87; 95% CI, 0.64 to 1.19; P = 0.38). No significant between-trial heterogeneity was found for these analyses (I2 = 0). Reduced mortality rates almost achieved statistical significance when the results of all included studies (RCTs and cohort studies) were pooled (RR, 0.83; 95% CI, 0.69 to 1.00; P = 0.054).
Conclusions: Pooled results from small RCTs suggest reduced clinical failure rates and intensive care unit length-of-stay when using continuous/extended infusions of antibiotics in critically ill patients. Reduced mortality rates almost achieved statistical significance when the results of RCTs were combined with cohort studies. These results support the conduct of adequately powered RCTs to define better the utility of continuous/extended infusions in the era of antibiotic resistance.
Figures






References
-
- Schentag JJ, Nix DE, Adelman MH. Mathematical examination of dual individualization principles (I): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin: DICP. Ann Pharmacother. 1991;17:1050–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

