Translational profiling in childhood acute lymphoblastic leukemia: no evidence for glucocorticoid regulation of mRNA translation
- PMID: 24289529
- PMCID: PMC4046653
- DOI: 10.1186/1471-2164-14-844
Translational profiling in childhood acute lymphoblastic leukemia: no evidence for glucocorticoid regulation of mRNA translation
Abstract
Background: Glucocorticoids (GCs) are natural stress induced steroid hormones causing cell cycle arrest and cell death in lymphoid tissues. Therefore they are the central component in the treatment of lymphoid malignancies, in particular childhood acute lymphoblastic leukemia (chALL). GCs act mainly via regulating gene transcription, which has been intensively studied by us and others. GC control of mRNA translation has also been reported but has never been assessed systematically. In this study we investigate the effect of GCs on mRNA translation on a genome-wide scale.
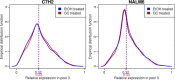
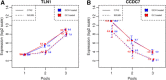
Results: Childhood T- (CCRF-CEM) and precursor B-ALL (NALM6) cells were exposed to GCs and subjected to "translational profiling", a technique combining sucrose-gradient fractionation followed by Affymetrix Exon microarray analysis of mRNA from different fractions, to assess the translational efficiency of the expressed genes. Analysis of GC regulation in ribosome-bound fractions versus transcriptional regulation revealed no significant differences, i.e., GC did not entail a significant shift between ribosomal bound and unbound mRNAs.
Conclusions: In the present study we analyzed for the first time possible effects of GC on the translational efficiency of expressed genes in two chALL model systems employing whole genome polysome profiling. Our results did not reveal significant differences in translational efficiency of expressed genes thereby arguing against a potential widespread regulatory effect of GCs on translation at least in the investigated in vitro systems.
Figures

References
-
- Laudet V, Gronemeyer H. The Nuclear Receptor Facts book. London, UK: Academic Press; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

