Activation of microglia induces symptoms of Parkinson's disease in wild-type, but not in IL-1 knockout mice
- PMID: 24289537
- PMCID: PMC4220804
- DOI: 10.1186/1742-2094-10-143
Activation of microglia induces symptoms of Parkinson's disease in wild-type, but not in IL-1 knockout mice
Abstract
Background: Parkinson's disease (PD) is an age-related progressive neurodegenerative disorder caused by selective loss of dopaminergic neurons from the substantia nigra (SN) to the striatum. The initial factor that triggers neurodegeneration is unknown; however, inflammation has been demonstrated to be significantly involved in the progression of PD. The present study was designed to investigate the role of the pro-inflammatory cytokine interleukin-1 (IL-1) in the activation of microglia and the decline of motor function using IL-1 knockout (KO) mice.
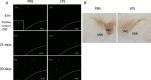
Methods: Lipopolysaccharide (LPS) was stereotaxically injected into the SN of mice brains as a single dose or a daily dose for 5 days (5 mg/2 ml/injection, bilaterally). Animal behavior was assessed with the rotarod test at 2 hr and 8, 15 and 22 days after the final LPS injection.
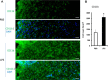
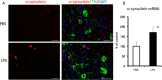
Results: LPS treatment induced the activation of microglia, as demonstrated by production of IL-1β and tumor necrosis factor (TNF) α as well as a change in microglial morphology. The number of cells immunoreactive for 4-hydroxynonenal (4HNE) and nitrotyrosine (NT), which are markers for oxidative insults, increased in the SN, and impairment of motor function was observed after the subacute LPS treatment. Cell death and aggregation of α-synuclein were observed 21 and 30 days after the final LPS injection, respectively. Behavioral deficits were observed in wild-type and TNFα KO mice, but IL-1 KO mice behaved normally. Tyrosine hydroxylase (TH) gene expression was attenuated by LPS treatment in wild-type and TNFα KO mice but not in IL-1 KO mice.
Conclusions: The subacute injection of LPS into the SN induces PD-like pathogenesis and symptoms in mice that mimic the progressive changes of PD including the aggregation of α-synuclein. LPS-induced dysfunction of motor performance was accompanied by the reduced gene expression of TH. These findings suggest that activation of microglia by LPS causes functional changes such as dopaminergic neuron attenuation in an IL-1-dependent manner, resulting in PD-like behavioral impairment.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

