Viral subversion of nucleocytoplasmic trafficking
- PMID: 24289861
- PMCID: PMC3910510
- DOI: 10.1111/tra.12137
Viral subversion of nucleocytoplasmic trafficking
Abstract
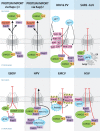
Trafficking of proteins and RNA into and out of the nucleus occurs through the nuclear pore complex (NPC). Because of its critical function in many cellular processes, the NPC and transport factors are common targets of several viruses that disrupt key constituents of the machinery to facilitate viral replication. Many viruses such as poliovirus and severe acute respiratory syndrome (SARS) virus inhibit protein import into the nucleus, whereas viruses such as influenza A virus target and disrupt host mRNA nuclear export. Current evidence indicates that these viruses may employ such strategies to avert the host immune response. Conversely, many viruses co-opt nucleocytoplasmic trafficking to facilitate transport of viral RNAs. As viral proteins interact with key regulators of the host nuclear transport machinery, viruses have served as invaluable tools of discovery that led to the identification of novel constituents of nuclear transport pathways. This review explores the importance of nucleocytoplasmic trafficking to viral pathogenesis as these studies revealed new antiviral therapeutic strategies and exposed previously unknown cellular mechanisms. Further understanding of nuclear transport pathways will determine whether such therapeutics will be useful treatments for important human pathogens.
Keywords: mRNA export; nuclear import; nuclear pore complex; nuclear transport; nucleocytoplasmic trafficking; virus.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

References
-
- Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue‐specific and developmental functions. Nat Rev Mol Cell Biol 2012;13:687–699. - PubMed
-
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 2003;4:775–789. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

