Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer
- PMID: 24290911
- PMCID: PMC3902970
- DOI: 10.1016/j.immuni.2013.11.003
Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer
Abstract
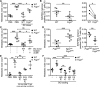
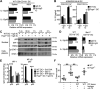
Cancers arising in mucosal tissues account for a disproportionately large fraction of malignancies. Immunoglobulin G (IgG) and the neonatal Fc receptor for IgG (FcRn) have an important function in the mucosal immune system that we have now shown extends to the induction of CD8(+) T cell-mediated antitumor immunity. We demonstrate that FcRn within dendritic cells (DCs) was critical for homeostatic activation of mucosal CD8(+) T cells that drove protection against the development of colorectal cancers and lung metastases. FcRn-mediated tumor protection was driven by DCs activation of endogenous tumor-reactive CD8(+) T cells via the cross-presentation of IgG complexed antigens (IgG IC), as well as the induction of cytotoxicity-promoting cytokine secretion, particularly interleukin-12, both of which were independently triggered by the FcRn-IgG IC interaction in murine and human DCs. FcRn thus has a primary role within mucosal tissues in activating local immune responses that are critical for priming efficient anti-tumor immunosurveillance.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



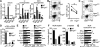

References
-
- Antonios D, Rousseau P, Larange A, Kerdine-Romer S, Pallardy M. Mechanisms of IL-12 Synthesis by Human Dendritic Cells Treated with the Chemical Sensitizer NiSO4. J. Immunol. 2010;185:89–98. - PubMed
-
- Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/delta716 Cdx2+/− compound mutant mice. Nat. Genet. 2003;35:323–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK044319/DK/NIDDK NIH HHS/United States
- R01 DK073338/DK/NIDDK NIH HHS/United States
- R01 DK088199/DK/NIDDK NIH HHS/United States
- DK47700/DK/NIDDK NIH HHS/United States
- R01 DK051362/DK/NIDDK NIH HHS/United States
- CAPMC/ CIHR/Canada
- R56 DK047700/DK/NIDDK NIH HHS/United States
- DK053162/DK/NIDDK NIH HHS/United States
- DK73338/DK/NIDDK NIH HHS/United States
- DK088199/DK/NIDDK NIH HHS/United States
- T32 DK007737/DK/NIDDK NIH HHS/United States
- R01 DK044319/DK/NIDDK NIH HHS/United States
- R01 DK047700/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- P30DK034854/DK/NIDDK NIH HHS/United States
- DK044319/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- DK53056/DK/NIDDK NIH HHS/United States
- R56 DK053056/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

