Tracking progesterone receptor-mediated actions in breast cancer
- PMID: 24291072
- PMCID: PMC3943696
- DOI: 10.1016/j.pharmthera.2013.11.010
Tracking progesterone receptor-mediated actions in breast cancer
Abstract
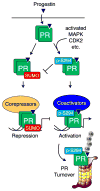
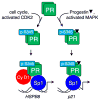
Ovarian steroid hormones contribute to breast cancer initiation and progression primarily through the actions of their nuclear transcription factors, the estrogen receptor alpha (ERα) and progesterone receptors (PRs). These receptors are important drivers of the luminal A and B subtypes of breast cancer, where estrogen-blocking drugs have been effective endocrine therapies for patients with these tumors. However, many patients do not respond, or become resistant to treatment. When endocrine therapies fail, the luminal subtypes of breast cancer are more difficult to treat because these subtypes are among the most heterogeneous in terms of mutation diversity and gene expression profiles. Recent evidence suggests that progestin and PR actions may be important drivers of luminal breast cancers. Clinical trial data has demonstrated that hormone replacement therapy with progestins drives invasive breast cancer and results in greater mortality. PR transcriptional activity is dependent upon cross-talk with growth factor signaling pathways that alter PR phosphorylation, acetylation, or SUMOylation as mechanisms for regulating PR target gene selection required for increased cell proliferation and survival. Site-specific PR phosphorylation is the primary driver of gene-selective PR transcriptional activity. However, PR phosphorylation and heightened transcriptional activity is coupled to rapid PR protein degradation; the range of active PR detected in tumors is likely to be dynamic. Thus, PR target gene signatures may provide a more accurate means of tracking PR's contribution to tumor progression rather than standard clinical protein-based (IHC) assays. Further development of antiprogestin therapies should be considered alongside antiestrogens and aromatase inhibitors.
Keywords: Antiprogestins; Estrogen receptor (ER); Gene expression; Phosphorylation; Progesterone receptor (PR); SUMOylation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Carol A. Lange is a consultant for Arno Therapeutics, Inc. (Flemington, NJ). Todd P. Knutson declares no conflicts of interest.
Figures
References
-
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–33956. - PubMed
-
- Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol 2012 - PMC - PubMed
-
- Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med. 2000;342:564–571. - PubMed
-
- Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146:3577–3588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

