FK506-binding protein 1b/12.6: a key to aging-related hippocampal Ca2+ dysregulation?
- PMID: 24291098
- PMCID: PMC4037392
- DOI: 10.1016/j.ejphar.2013.10.070
FK506-binding protein 1b/12.6: a key to aging-related hippocampal Ca2+ dysregulation?
Abstract
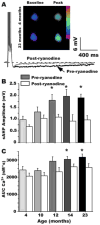
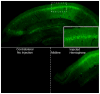
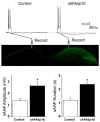
It has been recognized for some time that the Ca(2+)-dependent slow afterhyperpolarization (sAHP) is larger in hippocampal neurons of aged compared with young animals. In addition, extensive studies since have shown that other Ca(2+)-mediated electrophysiological responses are increased in hippocampus with aging, including Ca(2+) transients, L-type voltage-gated Ca(2+) channel activity, Ca(2+) spike duration and action potential accommodation. Elevated Ca(2+)-induced Ca(2+) release from ryanodine receptors (RyRs) appears to drive amplification of the Ca(2+) responses. Components of this Ca(2+) dysregulation phenotype correlate with deficits in cognitive function and plasticity, indicating they may play critical roles in aging-related impairment of brain function. However, the molecular mechanisms underlying aging-related Ca(2+) dysregulation are not well understood. FK506-binding proteins 1a and 1b (FKBP1a/1b, also known as FKBP12/12.6) are immunophilin proteins that bind the immunosuppressant drugs FK506 and rapamycin. In muscle cells, FKBP1a/1b also bind RyRs and inhibits Ca(2+)-induced Ca(2+) release, but it is not clear whether FKBPs act similarly in brain cells. Recently, we found that selectively disrupting hippocampal FKBP1b function in young rats, either by microinjecting adeno-associated viral vectors expressing siRNA, or by treatment with rapamycin, increases the sAHP and recapitulates much of the hippocampal Ca(2+) dysregulation phenotype. Moreover, in microarray studies, we found FKBP1b gene expression was downregulated in hippocampus of aging rats and early-stage Alzheimer's disease subjects. These results suggest the novel hypothesis that declining FKBP function is a key factor in aging-related Ca(2+) dysregulation in the brain and point to potential new therapeutic targets for counteracting unhealthy brain aging.
Keywords: Aging; Calcium; FKBP1b; Ryanodine receptor.
© 2013 Published by Elsevier B.V.
Figures




References
-
- Aitken DH, Meaney MJ. Temporally graded, age-related impairments in spatial memory in the rat. Neurobiol Aging. 1989;10:273–276. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5280–5285. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

