Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: an analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve)
- PMID: 24291272
- PMCID: PMC3962709
- DOI: 10.1016/j.jacc.2013.10.057
Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: an analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve)
Abstract
Objectives: The goal of this study was to determine whether a less-invasive approach to aortic valve replacement (AVR) improves clinical outcomes in diabetic patients with aortic stenosis (AS).
Background: Diabetes is associated with increased morbidity and mortality after surgical AVR for AS.
Methods: Among treated patients with severe symptomatic AS at high risk for surgery in the PARTNER (Placement of Aortic Transcatheter Valve) trial, we examined outcomes stratified according to diabetes status of patients randomly assigned to receive transcatheter or surgical AVR. The primary outcome was all-cause mortality at 1 year.
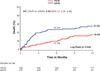
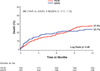
Results: Among 657 patients enrolled in PARTNER who underwent treatment, there were 275 patients with diabetes (145 transcatheter, 130 surgical). There was a significant interaction between diabetes and treatment group for 1-year all-cause mortality (p = 0.048). Among diabetic patients, all-cause mortality at 1 year was 18.0% in the transcatheter group and 27.4% in the surgical group (hazard ratio: 0.60 [95% confidence interval: 0.36 to 0.99]; p = 0.04). Results were consistent among patients treated via transfemoral or transapical routes. In contrast, among nondiabetic patients, there was no significant difference in all-cause mortality at 1 year (p = 0.48). Among diabetic patients, the 1-year rates of stroke were similar between treatment groups (3.5% transcatheter vs. 3.5% surgery; p = 0.88), but the rate of renal failure requiring dialysis >30 days was lower in the transcatheter group (0% vs. 6.1%; p = 0.003).
Conclusions: Among patients with diabetes and severe symptomatic AS at high risk for surgery, this post-hoc stratified analysis of the PARTNER trial suggests there is a survival benefit, no increase in stroke, and less renal failure from treatment with transcatheter AVR compared with surgical AVR. (The PARTNER Trial: Placement of AoRTic TraNscathetER Valve Trial; NCT00530894).
Keywords: aortic stenosis; diabetes; transcatheter aortic valve replacement.
Copyright © 2014 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





Comment in
-
Who comes off best with closed chest? Aortic valve replacement in patients with high surgical risk.J Am Coll Cardiol. 2014 Mar 25;63(11):1110-1. doi: 10.1016/j.jacc.2013.10.059. Epub 2013 Nov 27. J Am Coll Cardiol. 2014. PMID: 24291269 No abstract available.
References
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–269. - PubMed
-
- Mak KH, Topol EJ. Emerging concepts in the management of acute myocardial infarction in patients with diabetes mellitus. J Am Coll Cardiol. 2000;35:563–588. - PubMed
-
- Falcao-Pires I, Hamdani N, Borbely A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1129. - PubMed
-
- Halkos ME, Kilgo P, Lattouf OM, et al. The effect of diabetes mellitus on in-hospital and long-term outcomes after heart valve operations. Ann Thorac Surg. 2010;90:124–130. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

