Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1
- PMID: 24291345
- PMCID: PMC4040342
- DOI: 10.1016/j.jim.2013.11.022
Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1
Abstract
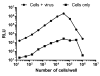
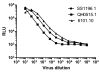
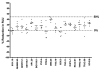
The TZM-bl assay measures antibody-mediated neutralization of HIV-1 as a function of reductions in HIV-1 Tat-regulated firefly luciferase (Luc) reporter gene expression after a single round of infection with Env-pseudotyped viruses. This assay has become the main endpoint neutralization assay used for the assessment of pre-clinical and clinical trial samples by a growing number of laboratories worldwide. Here we present the results of the formal optimization and validation of the TZM-bl assay, performed in compliance with Good Clinical Laboratory Practice (GCLP) guidelines. The assay was evaluated for specificity, accuracy, precision, limits of detection and quantitation, linearity, range and robustness. The validated manual TZM-bl assay was also adapted, optimized and qualified to an automated 384-well format.
Keywords: Assay validation; GCLP; HIV; Neutralizing antibody; TZM-bl cells.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
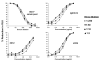




References
-
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. Journal of virology. 2004;78:13232–52. - PMC - PubMed
-
- Choudhry V, Zhang MY, Sidorov IA, Louis JM, Harris I, Dimitrov AS, Bouma P, Cham F, Choudhary A, Rybak SM, Fouts T, Montefiori DC, Broder CC, Quinnan GV, Jr, Dimitrov DS. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007;363:79–90. - PMC - PubMed
-
- Ezzelle J, Rodriguez-Chavez I, Darden J, Stirewalt M, Kunwar N, Hitchcock R, Walter T, D'souza M. Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. Journal of pharmaceutical and biomedical analysis. 2008;46:18–29. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

