Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24))
- PMID: 24291635
- PMCID: PMC3908837
- DOI: 10.1016/j.mce.2013.11.008
Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24))
Abstract
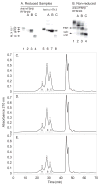
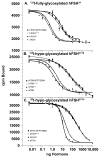
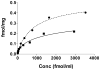
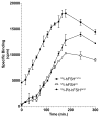
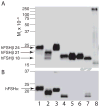
Hypo-glycosylated hFSH(21/18) (possesses FSHβ(21) and FSHβ(18)bands) was isolated from hLH preparations by immunoaffinity chromatography followed by gel filtration. Fully-glycosylated hFSH(24) was prepared by combining the fully-glycosylated FSHβ(24) variant with hCGα and isolating the heterodimer. The hFSH(21/18) glycoform preparation was significantly smaller than the hFSH(24) preparation and possessed 60% oligomannose glycans, which is unusual for hFSH. Hypo-glycosylated hFSH(21/18) was 9- to 26-fold more active than fully-glycosylated hFSH(24) in FSH radioligand assays. Significantly greater binding of (125)I-hFSH(21/18) tracer than hFSH(24) tracer was observed in all competitive binding assays. In addition, higher binding of hFSH(21/18) was noted in association and saturation binding assays, in which twice as much hFSH(21/18) was bound as hFSH(24). This suggests that more ligand binding sites are available to hFSH(21/18) in FSHR than to hFSH(24). Hypo-glycosylated hFSH(21/18) also bound rat FSHRs more rapidly, exhibiting almost no lag in binding, whereas hFSH(24) specific binding proceeded very slowly for almost the first hour of incubation.
Keywords: FSH isoforms; FSHR; Oligosaccharide.
Copyright © 2013 The Authors. Published by Elsevier Ireland Ltd.. All rights reserved.
Figures

References
-
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate Journal. 1999;16:1091–1123. - PubMed
-
- Andersen CY, Leonardsen L, Ulloa-Aquirre A, Barrios-de-Tomasi J, Kristensen KS, Byskov AG. Effect of different FSH isoforms on cyclic-AMP production by mouse cumulus-oocyte complexes: a time course study. Molecular Human Reproduction. 2001;7:129–135. - PubMed
-
- Bishop LA, Nguyen TV, Schofield PR. Both of the β-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136:2635–2640. - PubMed
-
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine–linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. J Mol Endocrinol. 1994;8:722–731. - PubMed
-
- Bousfield GR, Baker VL, Gotschall RR, Butnev VY, Butnev VY. Carbohydrate analysis of glycoprotein hormones. Methods. 2000;21:15–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

