Luminal epithelium in endometrial fragments affects their vascularization, growth and morphological development into endometriosis-like lesions in mice
- PMID: 24291760
- PMCID: PMC3917243
- DOI: 10.1242/dmm.013664
Luminal epithelium in endometrial fragments affects their vascularization, growth and morphological development into endometriosis-like lesions in mice
Abstract
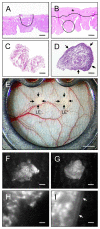
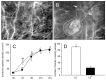
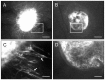
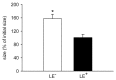
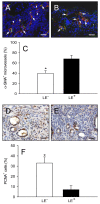
In endometriosis research, endometriosis-like lesions are usually induced in rodents by transplantation of isolated endometrial tissue fragments to ectopic sites. In the present study, we investigated whether this approach is affected by the cellular composition of the grafts. For this purpose, endometrial tissue fragments covered with luminal epithelium (LE(+)) and without luminal epithelium (LE(-)) were transplanted from transgenic green-fluorescent-protein-positive (GFP(+)) donor mice into the dorsal skinfold chamber of GFP(-) wild-type recipient animals to analyze their vascularization, growth and morphology by means of repetitive intravital fluorescence microscopy, histology and immunohistochemistry during a 14-day observation period. LE(-) fragments developed into typical endometriosis-like lesions with cyst-like dilated endometrial glands and a well-vascularized endometrial stroma. In contrast, LE(+) fragments exhibited a polypoid morphology and a significantly reduced blood perfusion after engraftment, because the luminal epithelium prevented the vascular interconnection with the microvasculature of the surrounding host tissue. This was associated with a markedly decreased growth rate of LE(+) lesions compared with LE(-) lesions. In addition, we found that many GFP(+) microvessels grew outside the LE(-) lesions and developed interconnections to the host microvasculature, indicating that inosculation is an important mechanism in the vascularization process of endometriosis-like lesions. Our findings demonstrate that the luminal epithelium crucially affects the vascularization, growth and morphology of endometriosis-like lesions. Therefore, it is of major importance to standardize the cellular composition of endometrial grafts in order to increase the validity and reliability of pre-clinical rodent studies in endometriosis research.
Keywords: Angiogenesis; Dorsal skinfold chamber; Endometriosis; Endometriotic lesion; Intravital fluorescence microscopy; Luminal epithelium; Morphology; Vascularization.
Figures


References
-
- Baker M., Wayland H. (1974). On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc. Res. 7, 131–143 - PubMed
-
- Feng D., Welker S., Körbel C., Rudzitis-Auth J., Menger M. D., Montenarh M., Laschke M. W. (2012). Protein kinase CK2 is a regulator of angiogenesis in endometriotic lesions. Angiogenesis 15, 243–252 - PubMed
-
- Gao X., Yeh Y. C., Outley J., Simon J., Botteman M., Spalding J. (2006). Health-related quality of life burden of women with endometriosis: a literature review. Curr. Med. Res. Opin. 22, 1787–1797 - PubMed
-
- Gipson I. K., Blalock T., Tisdale A., Spurr-Michaud S., Allcorn S., Stavreus-Evers A., Gemzell K. (2008). MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol. Reprod. 78, 134–142 - PubMed
-
- Groothuis P. G., Nap A. W., Winterhager E., Grümmer R. (2005). Vascular development in endometriosis. Angiogenesis 8, 147–156 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

