Hypoxia promotes liver-stage malaria infection in primary human hepatocytes in vitro
- PMID: 24291761
- PMCID: PMC3917242
- DOI: 10.1242/dmm.013490
Hypoxia promotes liver-stage malaria infection in primary human hepatocytes in vitro
Abstract
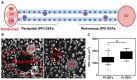
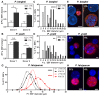
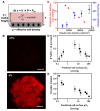
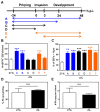
Homeostasis of mammalian cell function strictly depends on balancing oxygen exposure to maintain energy metabolism without producing excessive reactive oxygen species. In vivo, cells in different tissues are exposed to a wide range of oxygen concentrations, and yet in vitro models almost exclusively expose cultured cells to higher, atmospheric oxygen levels. Existing models of liver-stage malaria that utilize primary human hepatocytes typically exhibit low in vitro infection efficiencies, possibly due to missing microenvironmental support signals. One cue that could influence the infection capacity of cultured human hepatocytes is the dissolved oxygen concentration. We developed a microscale human liver platform comprised of precisely patterned primary human hepatocytes and nonparenchymal cells to model liver-stage malaria, but the oxygen concentrations are typically higher in the in vitro liver platform than anywhere along the hepatic sinusoid. Indeed, we observed that liver-stage Plasmodium parasite development in vivo correlates with hepatic sinusoidal oxygen gradients. Therefore, we hypothesized that in vitro liver-stage malaria infection efficiencies might improve under hypoxia. Using the infection of micropatterned co-cultures with Plasmodium berghei, Plasmodium yoelii or Plasmodium falciparum as a model, we observed that ambient hypoxia resulted in increased survival of exo-erythrocytic forms (EEFs) in hepatocytes and improved parasite development in a subset of surviving EEFs, based on EEF size. Further, the effective cell surface oxygen tensions (pO2) experienced by the hepatocytes, as predicted by a mathematical model, were systematically perturbed by varying culture parameters such as hepatocyte density and height of the medium, uncovering an optimal cell surface pO2 to maximize the number of mature EEFs. Initial mechanistic experiments revealed that treatment of primary human hepatocytes with the hypoxia mimetic, cobalt(II) chloride, as well as a HIF-1α activator, dimethyloxalylglycine, also enhance P. berghei infection, suggesting that the effect of hypoxia on infection is mediated in part by host-dependent HIF-1α mechanisms.
Keywords: Hypoxia; Liver-stage malaria; Primary hepatocytes.
Figures
References
-
- Allen J. W., Bhatia S. N. (2003). Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol. Bioeng. 82, 253–262 - PubMed
-
- Allen J. W., Khetani S. R., Bhatia S. N. (2005). In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci. 84, 110–119 - PubMed
-
- Arrais-Silva W. W., Pinto E. F., Rossi-Bergmann B., Giorgio S. (2006). Hyperbaric oxygen therapy reduces the size of Leishmania amazonensis-induced soft tissue lesions in mice. Acta Trop. 98, 130–136 - PubMed
-
- Bhatia S. N., Toner M., Foy B. D., Rotem A., O’Neil K. M., Tompkins R. G., Yarmush M. L. (1996). Zonal liver cell heterogeneity: effects of oxygen on metabolic functions of hepatocytes. J. Cell. Eng. 1, 125–135
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

