Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions
- PMID: 24292009
- PMCID: PMC4229038
- DOI: 10.1007/s00401-013-1220-8
Early loss of oligodendrocytes in human and experimental neuromyelitis optica lesions
Abstract
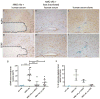
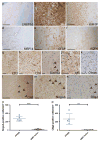
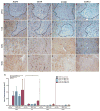
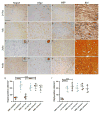
Neuromyelitis optica (NMO) is a chronic, mostly relapsing inflammatory demyelinating disease of the CNS characterized by serum anti-aquaporin 4 (AQP4) antibodies in the majority of patients. Anti-AQP4 antibodies derived from NMO patients target and deplete astrocytes in experimental models when co-injected with complement. However, the time course and mechanisms of oligodendrocyte loss and demyelination and the fate of oligodendrocyte precursor cells (OPC) have not been examined in detail. Also, no studies regarding astrocyte repopulation of experimental NMO lesions have been reported. We utilized two rat models using either systemic transfer or focal intracerebral injection of recombinant human anti-AQP4 antibodies to generate NMO-like lesions. Time-course experiments were performed to examine oligodendroglial and astroglial damage and repair. In addition, oligodendrocyte pathology was studied in early human NMO lesions. Apart from early complement-mediated astrocyte destruction, we observed a prominent, very early loss of oligodendrocytes and oligodendrocyte precursor cells (OPCs) as well as a delayed loss of myelin. Astrocyte repopulation of focal NMO lesions was already substantial after 1 week. Olig2-positive OPCs reappeared before NogoA-positive, mature oligodendrocytes. Thus, using two experimental models that closely mimic the human disease, our study demonstrates that oligodendrocyte and OPC loss is an extremely early feature in the formation of human and experimental NMO lesions and leads to subsequent, delayed demyelination, highlighting an important difference in the pathogenesis of MS and NMO.
Conflict of interest statement
Figures





References
-
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66(5):617–629. doi: 10.1002/ana.21802. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

