A recombinant influenza virus vaccine expressing the F protein of respiratory syncytial virus
- PMID: 24292020
- PMCID: PMC4013198
- DOI: 10.1007/s00705-013-1932-z
A recombinant influenza virus vaccine expressing the F protein of respiratory syncytial virus
Abstract
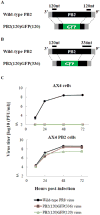
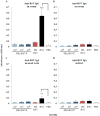
Infections with influenza and respiratory syncytial virus (RSV) rank high among the most common human respiratory diseases worldwide. Previously, we developed a replication-incompetent influenza virus by replacing the coding sequence of the PB2 gene, which encodes one of the viral RNA polymerase subunits, with that of a reporter gene. Here, we generated a PB2-knockout recombinant influenza virus expressing the F protein of RSV (PB2-RSVF virus) and tested its potential as a bivalent vaccine. In mice intranasally immunized with the PB2-RSVF virus, we detected high levels of antibodies against influenza virus, but not RSV. PB2-RSVF virus-immunized mice were protected from a lethal challenge with influenza virus but experienced severe body weight loss when challenged with RSV, indicating that PB2-RSVF vaccination enhanced RSV-associated disease. These results highlight one of the difficulties of developing an effective bivalent vaccine against influenza virus and RSV infections.
Figures
Similar articles
-
Influenza virus vaccine expressing fusion and attachment protein epitopes of respiratory syncytial virus induces protective antibodies in BALB/c mice.Antiviral Res. 2014 Apr;104:110-7. doi: 10.1016/j.antiviral.2014.01.022. Epub 2014 Feb 6. Antiviral Res. 2014. PMID: 24509239
-
Filling two needs with one deed: a combinatory mucosal vaccine against influenza A virus and respiratory syncytial virus.Front Immunol. 2024 Jun 21;15:1376395. doi: 10.3389/fimmu.2024.1376395. eCollection 2024. Front Immunol. 2024. PMID: 38975350 Free PMC article.
-
A Bivalent Vaccine Based on a PB2-Knockout Influenza Virus Protects Mice From Secondary Pneumococcal Pneumonia.J Infect Dis. 2015 Dec 15;212(12):1939-48. doi: 10.1093/infdis/jiv341. Epub 2015 Jun 29. J Infect Dis. 2015. PMID: 26123562 Free PMC article.
-
An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines.Virus Res. 1994 Apr;32(1):13-36. doi: 10.1016/0168-1702(94)90059-0. Virus Res. 1994. PMID: 8030364 Review.
-
What is the role of virus vaccination in patients with asthma?Curr Allergy Asthma Rep. 2007 Apr;7(1):72-6. doi: 10.1007/s11882-007-0033-z. Curr Allergy Asthma Rep. 2007. PMID: 17504664 Free PMC article. Review.
Cited by
-
Development and applications of single-cycle infectious influenza A virus (sciIAV).Virus Res. 2016 May 2;216:26-40. doi: 10.1016/j.virusres.2015.07.013. Epub 2015 Jul 26. Virus Res. 2016. PMID: 26220478 Free PMC article. Review.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
-
Stimulation of alpha2-adrenergic receptors impairs influenza virus infection.Sci Rep. 2018 Mar 15;8(1):4631. doi: 10.1038/s41598-018-22927-0. Sci Rep. 2018. PMID: 29545586 Free PMC article.
-
Reverse Genetics Approaches for the Development of Influenza Vaccines.Int J Mol Sci. 2016 Dec 22;18(1):20. doi: 10.3390/ijms18010020. Int J Mol Sci. 2016. PMID: 28025504 Free PMC article. Review.
-
Protection against respiratory syncytial virus by inactivated influenza virus carrying a fusion protein neutralizing epitope in a chimeric hemagglutinin.Nanomedicine. 2016 Apr;12(3):759-770. doi: 10.1016/j.nano.2015.11.007. Epub 2015 Dec 2. Nanomedicine. 2016. PMID: 26656630 Free PMC article.
References
-
- World healt organization. Acute Respiratory Infections. 2009 (Update September 2009) http://www.who.int/vaccine_research/diseases/ari/en/index2.html.
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. - PubMed
-
- Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced Disease and Pulmonary Eosinophilia Associated with Formalin-Inactivated Respiratory Syncytial Virus Vaccination Are Linked to G Glycoprotein CX3C-CX3CR1 Interaction and Expression of Substance P. Journal of Virology. 2003;77:9831–9844. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

