Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion
- PMID: 24292068
- PMCID: PMC3893890
- DOI: 10.1038/nchembio.1388
Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion
Abstract
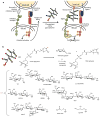
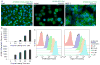
The increase of cell surface sialic acid is a characteristic shared by many tumor types. A correlation between hypersialylation and immunoprotection has been observed, but few hypotheses have provided a mechanistic understanding of this immunosuppressive phenomenon. Here, we show that increasing sialylated glycans on cancer cells inhibits human natural killer (NK) cell activation through the recruitment of sialic acid-binding immunoglobulin-like lectin 7 (Siglec-7). Key to these findings was the use of glycopolymers end-functionalized with phospholipids, which enable the introduction of synthetically defined glycans onto cancer cell surfaces. Remodeling the sialylation status of cancer cells affected the susceptibility to NK cell cytotoxicity via Siglec-7 engagement in a variety of tumor types. These results support a model in which hypersialylation offers a selective advantage to tumor cells under pressure from NK immunosurveillance by increasing Siglec ligands. We also exploited this finding to protect allogeneic and xenogeneic primary cells from NK-mediated killing, suggesting the potential of Siglecs as therapeutic targets in cell transplant therapy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



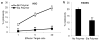
Comment in
-
Immunology: glyco-engineering 'super-self'.Nat Chem Biol. 2014 Jan;10(1):7-8. doi: 10.1038/nchembio.1415. Nat Chem Biol. 2014. PMID: 24346034 Free PMC article.
References
-
- Zamai L, et al. NK Cells and Cancer. J Immunol. 2007;178:4011–4016. - PubMed
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

