The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life
- PMID: 24292120
- PMCID: PMC3918677
- DOI: 10.1039/c3np70054b
The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life
Abstract
Covering: up to 2013. Although holo-acyl carrier protein synthase, AcpS, a phosphopantetheinyl transferase (PPTase), was characterized in the 1960s, it was not until the publication of the landmark paper by Lambalot et al. in 1996 that PPTases garnered wide-spread attention being classified as a distinct enzyme superfamily. In the past two decades an increasing number of papers have been published on PPTases ranging from identification, characterization, structure determination, mutagenesis, inhibition, and engineering in synthetic biology. In this review, we comprehensively discuss all current knowledge on this class of enzymes that post-translationally install a 4'-phosphopantetheine arm on various carrier proteins.
Figures


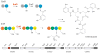














References
-
- Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. Chem Biol. 1996;3:923–936. - PubMed
-
- Crosby J, Crump MP. Nat Prod Rep. 2012;29:1111–1137. - PubMed
-
- Elovson J, Vagelos PR. J Biol Chem. 1968;243:3603–3611. - PubMed
-
- Walsh CT, Gehring AM, Weinreb PH, Quadri LEN, Flugel RS. Curr Opin Chem Biol. 1997;1:309–315. - PubMed
-
- Notredame C, Higgins DG, Heringa J. J Mol Biol. 2000;302:205–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

