Maternal hematopoietic TNF, via milk chemokines, programs hippocampal development and memory
- PMID: 24292233
- PMCID: PMC6169993
- DOI: 10.1038/nn.3596
Maternal hematopoietic TNF, via milk chemokines, programs hippocampal development and memory
Abstract
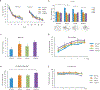
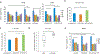
Tumor necrosis factor α (TNF) is a proinflammatory cytokine with established roles in host defense and immune system organogenesis. We studied TNF function and found a previously unidentified physiological function that extends its effect beyond the host into the developing offspring. A partial or complete maternal TNF deficit, specifically in hematopoietic cells, resulted in reduced milk levels of the chemokines IP-10, MCP-1, MCP-3, MCP-5 and MIP-1β, which in turn augmented offspring postnatal hippocampal proliferation, leading to improved adult spatial memory in mice. These effects were reproduced by the postpartum administration of a clinically used anti-TNF agent. Chemokines, fed to suckling pups of TNF-deficient mothers, restored both postnatal proliferation and spatial memory to normal levels. Our results identify a TNF-dependent 'lactrocrine' pathway that programs offspring hippocampal development and memory. The level of ambient TNF is known to be downregulated by physical activity, exercise and adaptive stress. We propose that the maternal TNF-milk chemokine pathway evolved to promote offspring adaptation to post-weaning environmental challenges and competition.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interests.
Figures


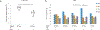
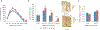
Comment in
-
Mother's milk programs offspring's cognition.Nat Neurosci. 2014 Jan;17(1):8-9. doi: 10.1038/nn.3611. Nat Neurosci. 2014. PMID: 24369371 No abstract available.
References
-
- Pasparakis M, Alexopoulou L, Episkopou V & Kollias G Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. The Journal of experimental medicine 184, 1397–1411 (1996). - PMC - PubMed
-
- Kuprash DV, et al. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. European journal of immunology 35, 1592–1600 (2005). - PubMed
-
- Muller N & Ackenheil M Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 22, 1–33 (1998). - PubMed
-
- Stellwagen D & Malenka RC Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059 (2006). - PubMed
-
- Golan H, Levav T, Mendelsohn A & Huleihel M Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex 14, 97–105 (2004). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

