Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies
- PMID: 24292404
- PMCID: PMC3915083
- DOI: 10.1007/s10585-013-9626-1
Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies
Abstract
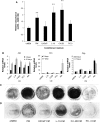
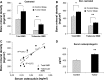
Castration-resistant prostate cancer (CRPC) is strongly associated with sclerotic bone metastases and poor prognosis. Models that mimic human CRPC are needed to identify the mechanisms for prostate cancer (PC) growth in bone and to develop new therapeutic strategies. We characterize a new model, LNCaP-19, and investigate the interaction between tumor cells and osteoblasts in the sclerotic tumor response of CRPC. Osteogenic profiling of PC cell lines (LNCaP-19, LNCaP, C4-2B4, and PC-3) was performed by gene expression arrays and mineral staining. Conditioned medium from MC3T3-E1 was used for osteoblast stimulation of CRPC cells. The capacity of LNCaP-19 cells to induce sclerotic lesions was assessed in intratibial xenografts and verified by serum markers, histological analysis and bone mineral density (BMD) measurements. The CRPC cell line LNCaP-19 expresses a pronounced osteogenic profile compared to its parental androgen-dependent cell line LNCaP. Osteoblast-derived factors further increase the expression of genes known to enhance metastatic progression of PC. LNCaP-19 forms sclerotic tumors in tibia of castrated mice as evident by increased total BMD (P < 0.01). There was a strong correlation between serum osteocalcin and BMD (total: R (2) 0.811, P < 0.01, trabecular: R (2) 0.673, P < 0.05). For the first time we demonstrate that a CRPC cell line generated in vitro has osteogenic capacity and that osteomimicry can be an inherent feature of these cells. Osteoblast-derived factors further promote the osteogenic and metastatic phenotype in CRPC cells. Altogether, our model demonstrates that both tumor cells and osteoblasts are mediators of the bone forming process of CRPC.
Figures


References
-
- Oefelein MG, Ricchuiti V, Conrad W, Seftel A, Bodner D, Goldman H, Resnick M. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166(5):1724–1728. doi: 10.1016/S0022-5347(05)65661-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

