New viruses for cancer therapy: meeting clinical needs
- PMID: 24292552
- PMCID: PMC4002503
- DOI: 10.1038/nrmicro3140
New viruses for cancer therapy: meeting clinical needs
Abstract
Early-stage clinical trials of oncolytic virotherapy have reported the safety of several virus platforms, and viruses from three families have progressed to advanced efficacy trials. In addition, preclinical studies have established proof-of-principle for many new genetic engineering strategies. Thus, the virotherapy field now has available a diverse collection of viruses that are equipped to address unmet clinical needs owing to improved systemic administration, greater tumour specificity and enhanced oncolytic efficacy. The current key challenge for the field is to develop viruses that replicate with greater efficiency within tumours while achieving therapeutic synergy with currently available treatments.
Figures
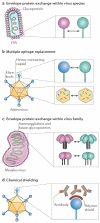
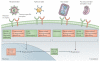
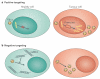
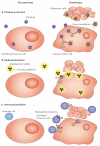
References
-
- Dock G. The influence of complicating diseases upon leukemia. Am. J. Med. Sci. 1904;127:563–592.
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. - PubMed
-
This paper provides a narrative introduction to the history of oncolytic virotherapy.
-
- Liu T-C, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature Rev. Clin. Oncol. 2007;4:101–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

