Efficacy of subcutaneous interferon β-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes
- PMID: 24292999
- PMCID: PMC4033030
- DOI: 10.1136/jnnp-2013-306289
Efficacy of subcutaneous interferon β-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes
Abstract
Aim: The REbif FLEXible dosing in early MS (REFLEX) study compared several brain MRI outcomes in patients presenting with clinically isolated syndromes suggestive of multiple sclerosis and treated with two dose-frequencies of subcutaneous interferon (IFN) β-1a or placebo.
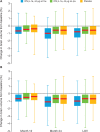
Methods: Patients were randomised (1:1:1) to IFN β-1a, 44 µg subcutaneously three times a week or once a week, or placebo three times a week for up to 24 months. MRI scans were performed every 3 months, or every 6 months if the patient developed clinically definite multiple sclerosis. End points analysed included: number of combined unique active lesions per patient per scan; numbers and volumes of new T2, T1 hypointense and gadolinium-enhancing (Gd+) lesions per patient per scan; and brain volume.
Results: 517 patients were randomised (intent-to-treat population: subcutaneous IFN β-1a three times a week, n=171; subcutaneous IFN β-1a once a week, n=175; placebo, n=171). Combined unique active lesions were lower in patients treated with subcutaneous IFN β-1a versus placebo (mean (SD) lesions per patient per scan: three times a week 0.6 (1.15); once a week 1.23 (4.26); placebo 2.70 (5.23); reduction versus placebo: three times a week 81%; once a week 63%; p<0.001) and with three times a week versus once a week (48% reduction; p=0.002). The mean numbers of new T2, T1 hypointense and Gd+ lesions were all significantly lower in the two active treatment arms compared with placebo (p≤0.004 for three times a week or once a week) and in the three times a week group compared with once a week (p≤0.012).
Conclusions: Both subcutaneous IFN β-1a 44 µg regimens improved MRI outcomes versus placebo, with the three times a week regimen having a more pronounced effect than once a week dosing.
Trial registration: clinicaltrial.gov identifier, NCT00404352.
Keywords: INTERFERON; MRI; Multiple Sclerosis; Randomised Trials.
Figures
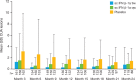
References
-
- Miller D, Barkhof F, Montalban X, et al. Clinically isolated syndromes suggestive of multiple sclerosis, part 1: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 2005;4:281–8 - PubMed
-
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 1. Clinical course and disability. Brain 1989; 112(Pt 1):133–46 - PubMed
-
- Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430–8 - PubMed
-
- Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009;374:1503–11 - PubMed
-
- Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000;343:898–904 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
