Ataxin-3 protein and RNA toxicity in spinocerebellar ataxia type 3: current insights and emerging therapeutic strategies
- PMID: 24293103
- PMCID: PMC4012159
- DOI: 10.1007/s12035-013-8596-2
Ataxin-3 protein and RNA toxicity in spinocerebellar ataxia type 3: current insights and emerging therapeutic strategies
Abstract
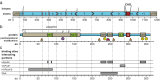
Ataxin-3 is a ubiquitously expressed deubiqutinating enzyme with important functions in the proteasomal protein degradation pathway and regulation of transcription. The C-terminus of the ataxin-3 protein contains a polyglutamine (PolyQ) region that, when mutationally expanded to over 52 glutamines, causes the neurodegenerative disease spinocerebellar ataxia 3 (SCA3). In spite of extensive research, the molecular mechanisms underlying the cellular toxicity resulting from mutant ataxin-3 remain elusive and no preventive treatment is currently available. It has become clear over the last decade that the hallmark intracellular ataxin-3 aggregates are likely not the main toxic entity in SCA3. Instead, the soluble PolyQ containing fragments arising from proteolytic cleavage of ataxin-3 by caspases and calpains are now regarded to be of greater influence in pathogenesis. In addition, recent evidence suggests potential involvement of a RNA toxicity component in SCA3 and other PolyQ expansion disorders, increasing the pathogenic complexity. Herein, we review the functioning of ataxin-3 and the involvement of known protein and RNA toxicity mechanisms of mutant ataxin-3 that have been discovered, as well as future opportunities for therapeutic intervention.
Figures
References
-
- Haberhausen G, Damian MS, Leweke F, Muller U. Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado–Joseph disease (MJD) J Neurol Sci. 1995;132:71–75. - PubMed
-
- Ranum LP, Lundgren JK, Schut LJ, Ahrens MJ, Perlman S, Aita J, Bird TD, Gomez C, Orr HT. Spinocerebellar ataxia type 1 and Machado–Joseph disease: incidence of CAG expansions among adult-onset ataxia patients from 311 families with dominant, recessive, or sporadic ataxia. Am J Hum Genet. 1995;57:603–608. - PMC - PubMed
-
- Silveira I, Lopes-Cendes I, Kish S, Maciel P, Gaspar C, Coutinho P, Botez MI, Teive H, Arruda W, Steiner CE, et al. Frequency of spinocerebellar ataxia type 1, dentatorubropallidoluysian atrophy, and Machado–Joseph disease mutations in a large group of spinocerebellar ataxia patients. Neurology. 1996;46:214–218. - PubMed
-
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord. 2012;27:1083–1091. - PubMed
-
- Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28:703–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

