Compound loss of muscleblind-like function in myotonic dystrophy
- PMID: 24293317
- PMCID: PMC3914532
- DOI: 10.1002/emmm.201303275
Compound loss of muscleblind-like function in myotonic dystrophy
Abstract
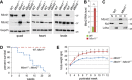
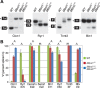
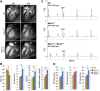
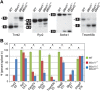
Myotonic dystrophy (DM) is a multi-systemic disease that impacts cardiac and skeletal muscle as well as the central nervous system (CNS). DM is unusual because it is an RNA-mediated disorder due to the expression of toxic microsatellite expansion RNAs that alter the activities of RNA processing factors, including the muscleblind-like (MBNL) proteins. While these mutant RNAs inhibit MBNL1 splicing activity in heart and skeletal muscles, Mbnl1 knockout mice fail to recapitulate the full-range of DM symptoms in these tissues. Here, we generate mouse Mbnl compound knockouts to test the hypothesis that Mbnl2 functionally compensates for Mbnl1 loss. Although Mbnl1(-/-) ; Mbnl2(-/-) double knockouts (DKOs) are embryonic lethal, Mbnl1(-/-) ; Mbnl2(+/-) mice are viable but develop cardinal features of DM muscle disease including reduced lifespan, heart conduction block, severe myotonia and progressive skeletal muscle weakness. Mbnl2 protein levels are elevated in Mbnl1(-/-) knockouts where Mbnl2 targets Mbnl1-regulated exons. These findings support the hypothesis that compound loss of MBNL function is a critical event in DM pathogenesis and provide novel mouse models to investigate additional pathways disrupted in this RNA-mediated disease.
Keywords: Mbnl1; Mbnl2; RNA-mediated disease; muscleblind-like; myotonic dystrophy.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures


References
-
- Fugier C, Klein AF, Hammer C, Vassilopoulos S, Ivarsson Y, Toussaint A, Tosch V, Vignaud A, Ferry A, Messaddeq N, et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat Med. 2011;17:720–725. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

