Loss of ATE1-mediated arginylation leads to impaired platelet myosin phosphorylation, clot retraction, and in vivo thrombosis formation
- PMID: 24293517
- PMCID: PMC3943321
- DOI: 10.3324/haematol.2013.093047
Loss of ATE1-mediated arginylation leads to impaired platelet myosin phosphorylation, clot retraction, and in vivo thrombosis formation
Abstract
Protein arginylation by arginyl-transfer RNA protein transferase (ATE1) is emerging as a regulator protein function that is reminiscent of phosphorylation. For example, arginylation of β-actin has been found to regulate lamellipodial formation at the leading edge in fibroblasts. This finding suggests that similar functions of β-actin in other cell types may also require arginylation. Here, we have tested the hypothesis that ATE1 regulates the cytoskeletal dynamics essential for in vivo platelet adhesion and thrombus formation. To test this hypothesis, we generated conditional knockout mice specifically lacking ATE1 in their platelets and in their megakaryocytes and analyzed the role of arginylation during platelet activation. Surprisingly, rather than finding an impairment of the actin cytoskeleton structure and its rearrangement during platelet activation, we observed that the platelet-specific ATE1 knockout led to enhanced clot retraction and in vivo thrombus formation. This effect might be regulated by myosin II contractility since it was accompanied by enhanced phosphorylation of the myosin regulatory light chain on Ser19, which is an event that activates myosin in vivo. Furthermore, ATE1 and myosin co-immunoprecipitate from platelet lysates. This finding suggests that these proteins directly interact within platelets. These results provide the first evidence that arginylation is involved in phosphorylation-dependent protein regulation, and that arginylation affects myosin function in platelets during clot retraction.
Figures
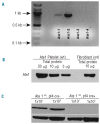
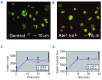
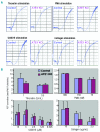
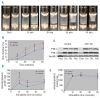

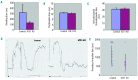
Comment in
-
Post-translational arginylation as a novel regulator of platelet function.Haematologica. 2014 Mar;99(3):402-4. doi: 10.3324/haematol.2013.103119. Haematologica. 2014. PMID: 24598850 Free PMC article. No abstract available.
References
-
- Kwon YT, Kashina AS, Davydov IV, Hu RG, An JY, Seo JW, et al. An essential role of N-terminal arginylation in cardiovascular development. Science. 2002;297(5578):96–9 - PubMed
-
- Carpio MA, Lopez Sambrooks C, Durand ES, Hallak ME. The arginylation-dependent association of calreticulin with stress granules is regulated by calcium. Biochem J. 2010;429(1):63. - PubMed
-
- Soffer RL. Enzymatic modification of proteins. V. Protein acceptor specificity in the arginine-transfer reaction. J Biol Chem. 1971;246(6):1602–6 - PubMed
-
- Ciechanover A, Ferber S, Ganoth D, Elias S, Hershko A, Arfin S. Purification and characterization of arginyl-tRNA-protein transferase from rabbit reticulocytes. Its involvement in post-translational modification and degradation of acidic NH2 termini substrates of the ubiquitin pathway. J Biol Chem. 1988;263(23):11155–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103533/GM/NIGMS NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
- P01 HL040387/HL/NHLBI NIH HHS/United States
- U01 HL099993/HL/NHLBI NIH HHS/United States
- R01 HL110110/HL/NHLBI NIH HHS/United States
- U01 HL099656/HL/NHLBI NIH HHS/United States
- P01 HL40387/HL/NHLBI NIH HHS/United States
- P01 HL064190/HL/NHLBI NIH HHS/United States
- R01 HL083392/HL/NHLBI NIH HHS/United States
- P01 HL120846/HL/NHLBI NIH HHS/United States
- P01 HL110860/HL/NHLBI NIH HHS/United States
- GM103533/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

