Endogenous β-glucocerebrosidase activity in Abca12⁻/⁻epidermis elevates ceramide levels after topical lipid application but does not restore barrier function
- PMID: 24293640
- PMCID: PMC3934733
- DOI: 10.1194/jlr.M044941
Endogenous β-glucocerebrosidase activity in Abca12⁻/⁻epidermis elevates ceramide levels after topical lipid application but does not restore barrier function
Abstract
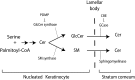
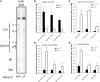
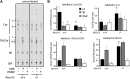
ABCA12 mutations disrupt the skin barrier and cause harlequin ichthyosis. We previously showed Abca12(-/-) skin has increased glucosylceramide (GlcCer) and correspondingly lower amounts of ceramide (Cer). To examine why loss of ABCA12 leads to accumulation of GlcCer, de novo sphingolipid synthesis was assayed using [(14)C]serine labeling in ex vivo skin cultures. A defect was found in β-glucocerebrosidase (GCase) processing of newly synthesized GlcCer species. This was not due to a decline in GCase function. Abca12(-/-) epidermis had 5-fold more GCase protein (n = 4, P < 0.01), and a 5-fold increase in GCase activity (n = 3, P < 0.05). As with Abca12(+/+) epidermis, immunostaining in null skin showed a typical interstitial distribution of the GCase protein in the Abca12(-/-) stratum corneum. Hence, we tested whether the block in GlcCer conversion could be circumvented by topically providing GlcCer. This approach restored up to 15% of the lost Cer products of GCase activity in the Abca12(-/-) epidermis. However, this level of barrier ceramide replacement did not significantly reduce trans-epidermal water loss function. Our results indicate loss of ABCA12 function results in a failure of precursor GlcCer substrate to productively interact with an intact GCase enzyme, and they support a model of ABCA12 function that is critical for transporting GlcCer into lamellar bodies.
Keywords: ABCA12 antibody; glucosylceramide; harlequin ichthyosis; skin permeability barrier.
Figures



Similar articles
-
Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes.J Biol Chem. 2009 Jul 10;284(28):18942-52. doi: 10.1074/jbc.M109.006973. Epub 2009 May 8. J Biol Chem. 2009. PMID: 19429679 Free PMC article.
-
Application of glucosylceramide-based liposomes increased the ceramide content in a three-dimensional cultured skin epidermis.Skin Pharmacol Physiol. 2014;27(1):18-24. doi: 10.1159/000351350. Epub 2013 Jul 24. Skin Pharmacol Physiol. 2014. PMID: 23887587
-
Defects in Stratum Corneum Desquamation Are the Predominant Effect of Impaired ABCA12 Function in a Novel Mouse Model of Harlequin Ichthyosis.PLoS One. 2016 Aug 23;11(8):e0161465. doi: 10.1371/journal.pone.0161465. eCollection 2016. PLoS One. 2016. PMID: 27551807 Free PMC article.
-
The roles of ABCA12 in epidermal lipid barrier formation and keratinocyte differentiation.Biochim Biophys Acta. 2014 Mar;1841(3):435-40. doi: 10.1016/j.bbalip.2013.08.009. Epub 2013 Aug 15. Biochim Biophys Acta. 2014. PMID: 23954554 Review.
-
Harlequin ichthyosis: ABCA12 mutations underlie defective lipid transport, reduced protease regulation and skin-barrier dysfunction.Cell Tissue Res. 2013 Feb;351(2):281-8. doi: 10.1007/s00441-012-1474-9. Epub 2012 Aug 4. Cell Tissue Res. 2013. PMID: 22864982 Review.
Cited by
-
The transcription cofactor CRTC1 protects from aberrant hepatic lipid accumulation.Sci Rep. 2016 Nov 21;6:37280. doi: 10.1038/srep37280. Sci Rep. 2016. PMID: 27869139 Free PMC article.
-
Efficacy and safety of secukinumab for the treatment of severe ABCA12 deficiency-related ichthyosis in a child.Skin Health Dis. 2021 May 3;1(2):e25. doi: 10.1002/ski2.25. eCollection 2021 Jun. Skin Health Dis. 2021. PMID: 35664977 Free PMC article.
-
Strategies to gain novel Alzheimer's disease diagnostics and therapeutics using modulators of ABCA transporters.Free Neuropathol. 2021;2:33. doi: 10.17879/freeneuropathology-2021-3528. Epub 2021 Dec 13. Free Neuropathol. 2021. PMID: 34977908 Free PMC article.
-
Human genetic defects of sphingolipid synthesis.J Inherit Metab Dis. 2025 Jan;48(1):e12745. doi: 10.1002/jimd.12745. Epub 2024 May 5. J Inherit Metab Dis. 2025. PMID: 38706107 Free PMC article. Review.
References
-
- Akiyama M., Sugiyama-Nakagiri Y., Sakai K., McMillan J. R., Goto M., Arita K., Tsuji-Abe Y., Tabata N., Matsuoka K., Sasaki R., et al. 2005. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J. Clin. Invest. 115: 1777–1784 - PMC - PubMed
-
- Elias P. M., Fartasch M., Crumrine D., Behne M., Uchida Y., Holleran W. M. 2000. Origin of the corneocyte lipid envelope (CLE): observations in harlequin ichthyosis and cultured human keratinocytes. J. Invest. Dermatol. 115: 765–769 - PubMed
-
- Hsu W. Y., Chen J. Y., Lin W. L., Tsay C. H. 1989. Harlequin fetus–a case report [article in Chinese]. Zhonghua Yi Xue Za Zhi (Taipei). 43: 63–66 - PubMed
-
- Moreau S., Salame E., Goullet de Rugy M., Delmas P. 1999. Harlequin fetus: a case report. Surg. Radiol. Anat. 21: 215–216 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

