Intermittent hypoxia exacerbates pancreatic β-cell dysfunction in a mouse model of diabetes mellitus
- PMID: 24293759
- PMCID: PMC3825434
- DOI: 10.5665/sleep.3214
Intermittent hypoxia exacerbates pancreatic β-cell dysfunction in a mouse model of diabetes mellitus
Abstract
Study objectives: The effects of intermittent hypoxia (IH) on pancreatic function in the presence of diabetes and the underlying mechanisms are unclear. We hypothesized that IH would exacerbate pancreatic β-cell dysfunction and alter the fatty acids in the male Tallyho/JngJ (TH) mouse, a rodent model of type 2 diabetes.
Design: TH mice were exposed for 14 d to either 8 h of IH or intermittent air (IA), followed by an intraperitoneal glucose tolerance test (IPGTT) and tissue harvest. The effect of IH on insulin release was determined by using a β3-adrenergic receptor (AR) agonist.
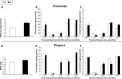
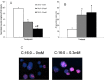
Measurements and results: During IH, pancreatic tissue pO2 decreased from 20.4 ± 0.9 to 5.7 ± 2.6 mm Hg, as determined by electron paramagnetic resonance oximetry. TH mice exposed to IH exhibited higher plasma glucose levels during the IPGTT (P < 0.001) while the insulin levels tended to be lower (P = 0.06). Pancreatic islets of the IH group showed an enhancement of the caspase-3 staining (P = 0.002). IH impaired the β-AR agonist-mediated insulin release (P < 0.001). IH increased the levels of the total free fatty acids and saturated fatty acids (palmitic and stearic acids), and decreased levels of the monounsaturated fatty acids in the pancreas and plasma. Ex vivo exposure of pancreatic islets to palmitic acid suppressed insulin secretion and decreased islet cell viability.
Conclusions: Intermittent hypoxia increases pancreatic apoptosis and exacerbates dysfunction in a polygenic rodent model of diabetes. An increase in free fatty acids and a shift in composition towards long chain saturated fatty acid species appear to mediate these effects.
Keywords: Intermittent hypoxia; diabetes mellitus; fatty acids; β-cell function.
Figures




References
-
- Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. - PubMed
-
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. - PubMed
-
- Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–6. - PubMed
-
- Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

