Neuropathic pain and cytokines: current perspectives
- PMID: 24294006
- PMCID: PMC3839806
- DOI: 10.2147/JPR.S53660
Neuropathic pain and cytokines: current perspectives
Abstract
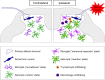
Neuropathic pain represents a major problem in clinical medicine because it causes debilitating suffering and is largely resistant to currently available analgesics. A characteristic of neuropathic pain is abnormal response to somatic sensory stimulation. Thus, patients suffering peripheral neuropathies may experience pain caused by stimuli which are normally nonpainful, such as simple touching of the skin or by changes in temperature, as well as exaggerated responses to noxious stimuli. Convincing evidence suggests that this hypersensitivity is the result of pain remaining centralized. In particular, at the first pain synapse in the dorsal horn of the spinal cord, the gain of neurons is increased and neurons begin to be activated by innocuous inputs. In recent years, it has become appreciated that a remote damage in the peripheral nervous system results in neuronal plasticity and changes in microglial and astrocyte activity, as well as infiltration of macrophages and T cells, which all contribute to central sensitization. Specifically, the release of pronociceptive factors such as cytokines and chemokines from neurons and non-neuronal cells can sensitize neurons of the first pain synapse. In this article we review the current evidence for the role of cytokines in mediating spinal neuron-non-neuronal cell communication in neuropathic pain mechanisms following peripheral nerve injury. Specific and selective control of cytokine-mediated neuronal-glia interactions results in attenuation of the hypersensitivity to both noxious and innocuous stimuli observed in neuropathic pain models, and may represent an avenue for future therapeutic intervention.
Keywords: anti-inflammatory cytokines; astrocytes; first pain synapse; microglia; proinflammatory cytokines.
Figures


References
-
- Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. - PubMed
-
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. - PubMed
-
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64(1):46–54. - PubMed
-
- Clark AK, Malcangio M. Microglial signalling mechanisms: Cathepsin S and Fractalkine. Exp Neurol. 2012;234(2):283–292. - PubMed
-
- Hansen RR, Malcangio M. Astrocytes-Multitaskers in chronic pain. Eur J Pharmacol. 2013;716(1–3):120–128. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

