Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons
- PMID: 24294191
- PMCID: PMC3827583
- DOI: 10.3389/fncel.2013.00209
Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons
Abstract
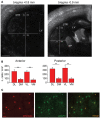
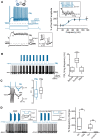
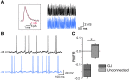
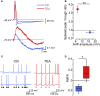
Fast-spiking interneurons (FSIs) play a central role in organizing the output of striatal neural circuits, yet functional interactions between these cells are still largely unknown. Here we investigated the interplay of action potential (AP) firing between electrically connected pairs of identified FSIs in mouse striatal slices. In addition to a loose coordination of firing activity mediated by membrane potential coupling, gap junctions (GJ) induced a frequency-dependent inhibition of spike discharge in coupled cells. At relatively low firing rates (2-20 Hz), some APs were tightly synchronized whereas others were inhibited. However, burst firing at intermediate frequencies (25-60 Hz) mostly induced spike inhibition, while at frequencies >50-60 Hz FSI pairs tended to synchronize. Spike silencing occurred even in the absence of GABAergic synapses or persisted after a complete block of GABAA receptors. Pharmacological suppression of presynaptic spike afterhyperpolarization (AHP) caused postsynaptic spikelets to become more prone to trigger spikes at near-threshold potentials, leading to a mostly synchronous firing activity. The complex pattern of functional coordination mediated by GJ endows FSIs with peculiar dynamic properties that may be critical in controlling striatal-dependent behavior.
Keywords: GABA; action potential; fast-spiking interneurons; gap junctions; striatum.
Figures




Similar articles
-
Functional properties of striatal fast-spiking interneurons.Front Syst Neurosci. 2011 Jun 20;5:45. doi: 10.3389/fnsys.2011.00045. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21743805 Free PMC article.
-
Frequency-dependent entrainment of striatal fast-spiking interneurons.J Neurophysiol. 2019 Sep 1;122(3):1060-1072. doi: 10.1152/jn.00369.2019. Epub 2019 Jul 17. J Neurophysiol. 2019. PMID: 31314645 Free PMC article.
-
Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance.J Neurosci. 2008 Oct 1;28(40):10075-80. doi: 10.1523/JNEUROSCI.2192-08.2008. J Neurosci. 2008. PMID: 18829965 Free PMC article.
-
Representation of the body in the lateral striatum of the freely moving rat: Fast Spiking Interneurons respond to stimulation of individual body parts.Brain Res. 2017 Feb 15;1657:101-108. doi: 10.1016/j.brainres.2016.11.033. Epub 2016 Nov 30. Brain Res. 2017. PMID: 27914882 Free PMC article.
-
Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4.J Neurophysiol. 2005 Jan;93(1):467-80. doi: 10.1152/jn.00520.2004. Epub 2004 Aug 18. J Neurophysiol. 2005. PMID: 15317837
Cited by
-
Transient Response of Basal Ganglia Network in Healthy and Low-Dopamine State.eNeuro. 2022 Mar 18;9(2):ENEURO.0376-21.2022. doi: 10.1523/ENEURO.0376-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35140075 Free PMC article.
-
Sex steroid hormones, the estrous cycle, and rapid modulation of glutamatergic synapse properties in the striatal brain regions with a focus on 17β-estradiol and the nucleus accumbens.Steroids. 2024 Jan;201:109344. doi: 10.1016/j.steroids.2023.109344. Epub 2023 Nov 17. Steroids. 2024. PMID: 37979822 Free PMC article. Review.
-
Desynchronization of fast-spiking interneurons reduces β-band oscillations and imbalance in firing in the dopamine-depleted striatum.J Neurosci. 2015 Jan 21;35(3):1149-59. doi: 10.1523/JNEUROSCI.3490-14.2015. J Neurosci. 2015. PMID: 25609629 Free PMC article.
-
The microcircuits of striatum in silico.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9554-9565. doi: 10.1073/pnas.2000671117. Epub 2020 Apr 22. Proc Natl Acad Sci U S A. 2020. PMID: 32321828 Free PMC article.
-
Preserving cortico-striatal function: deep brain stimulation in Huntington's disease.Front Syst Neurosci. 2015 Mar 11;9:32. doi: 10.3389/fnsys.2015.00032. eCollection 2015. Front Syst Neurosci. 2015. PMID: 25814939 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

