EGFR mutation testing on cytological and histological samples in non-small cell lung cancer: a Polish, single institution study and systematic review of European incidence
- PMID: 24294366
- PMCID: PMC3843260
EGFR mutation testing on cytological and histological samples in non-small cell lung cancer: a Polish, single institution study and systematic review of European incidence
Abstract
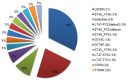
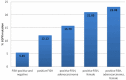
The targeted treatment of advanced non-small-cell lung cancer (NSCLC) depends on confirmation of activating somatic EGFR mutation. The aim of the study was to evaluate the incidence of EGFR mutations in NSCLC detected in cytological and histological material and present literature review on European EGFR mutation incidence. 273 patients with confirmed NSCLC were entered into the study: 189 histological, paraffin-embedded materials, 12 fresh and 72 fixed cytological specimens. DNA was extracted from both types of material and the EGFR mutation in exons 18-21 was analyzed by direct sequencing. In addition the EGFR gene copy number in cases with sufficient histological material (110 patients) was evaluated by fluorescent in situ hybridization (FISH) technique. The percentage of EGFR somatic mutations was 10.62%. FISH positive results (amplification or high polysomy of EGFR gene) were identified in 33 patients (30.0%). The strongest clinicopathological correlation with the EGFR mutation was found for histological type (adenocarcinoma; p < 0.01), gender (females; p < 0.01) and FISH positive result (p < 0.05). This is the first, single institution study that estimates the EGFR mutation incidence in the Polish population. Cytological material recovered from fixed preparations and stained with hematoxylin and eosin showed DNA quality comparable to fresh tumor cells and histological samples.
Keywords: EGFR amplification; EGFR mutation; cytology; non-small-cell lung cancer.
Figures


References
-
- Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. - PubMed
-
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. - PubMed
-
- Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
