Neoadjuvant hyperfractionated-accelerated radiotherapy with concomitant chemotherapy in esophageal cancer: phase II study
- PMID: 24294510
- PMCID: PMC3819777
- DOI: 10.3978/j.issn.2078-6891.2013.022
Neoadjuvant hyperfractionated-accelerated radiotherapy with concomitant chemotherapy in esophageal cancer: phase II study
Abstract
Purpose: Concomitant use of chemotherapy and a radiation dose schedule that is more efficient compared to conventional radiotherapy may provide better outcomes in patients with esophageal cancer. This study aimed to assess the efficacy and tolerability of neoadjuvant cisplatin-based chemotherapy and hyperfractionated accelerated radiotherapy regimen in this group of patients.
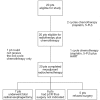
Methods and materials: A total of 20 newly diagnosed treatment-naïve esophageal cancer patients were included in the study. Neoadjuvant cisplatin and 5-FU were given with 28-day intervals in a total of three courses. Along with the third course of chemotherapy, hyperfractionated accelerated radiotherapy (HART) was given with the following dose schedule: 5760 cGy/36 fr/16 day.
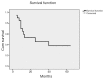
Results: All patients could receive the planned RT dose of 5760 cGy. Odynophagia was the most frequent grade III acute toxicity (50%). None of the acute toxicity reactions required treatment discontinuation. Grade III or higher subacute/late toxicity occurred in 10 patients (75%) including 5 deaths, mostly esophageal. Radiologically, 8 patients (40%) had complete response, 8 (40%) had partial response, and 3 (15%) had stable disease, with only 1 patient (5%) having progressive disease. Seven patients underwent surgery. Overall, 8 patients (40%) had local control. The 5 years overall survival rate was 38.1%.
Conclusions: Neoadjuvant hyperfractionated accelerated radiotherapy plus chemotherapy may help to target local disease control and increase survival in patients with esophageal cancer. Further studies to improve neoadjuvant and radical chemoradiotherapy dose schedules are warranted for maximum tumor control rates with minimal toxicity.
Keywords: Neoadjuvant radiochemotherapy; esophageal cancer; hyperfractionated-accelerated radiotherapy; safety; toxicity.
Figures
References
-
- Kasai M, Mori S, Watanabe T.Follow-up results after resection of thoracic esophageal carcinoma. World J Surg 1978;2:543-51 - PubMed
-
- Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg 1980;67:381-90 - PubMed
-
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43 - PubMed
-
- Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery 2005;137:172-7 - PubMed
LinkOut - more resources
Full Text Sources