A recombinant multiepitope protein for hepatitis B diagnosis
- PMID: 24294596
- PMCID: PMC3835477
- DOI: 10.1155/2013/148317
A recombinant multiepitope protein for hepatitis B diagnosis
Abstract
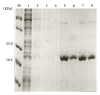
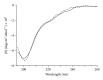
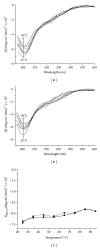
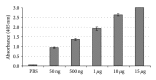
Hepatitis B is a liver inflammation caused by hepatitis B virus (HBV) and can be diagnosed in clinical stage by hepatitis B core antibody from IgM class (anti-HBcIgM). Hepatitis B core antibody from IgG class (Anti-HBcIgG) appears quickly after IgM, reaching high titers in chronic hepatitis, and remains even after cure. Since hepatitis B core antibody (anti-HBc) is the first antibody identified and sometimes the only marker detected during the course of infection, it can be used both to indicate HBV acute infection (anti-HBc-IgM) and to identify individuals who have come into contact with the virus (anti-HBc-IgG). In this work we propose a recombinant hepatitis B core multiepitope antigen (rMEHB) to be used for diagnosis of hepatitis B. For this purpose, a synthetic gene coding for rMEHB was designed and cloned into vector pET21a with a 6xHis tag at the C-terminal. Time course induction in E. coli showed an induced protein with an apparent molecular mass of ~21 kDa. Protein purification was performed by a single step with affinity chromatography Ni-NTA. Circular dichroism spectroscopy indicated rMEHB as a thermal stable protein at pH 7.0 and 8.0. In these conditions rMEHB was successfully used to perform an enzyme linked immuno sorbent assay (ELISA) with positive and negative sera.
Figures



References
-
- WHO. Prevention & Controlo of Viral Hepatitis Infection: Framework for Global Action. 2012, http://www.who.int/csr/disease/hepatitis/GHP_framework.pdf.
-
- Shi Y-H. Correlation between hepatitis B virus genotypes and clinical outcomes. Japan Journal Infection Diseases. 2012;65:476–482. - PubMed
-
- WHO. Global Alert and Response (GAR): Hepatitis B. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index3.html.
-
- Stuyver L, De Gendt S, Van Geyt C, et al. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. Journal of General Virology. 2000;81(1):67–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

