Tetrahydrobiopterin in cardiovascular health and disease
- PMID: 24294830
- PMCID: PMC4038990
- DOI: 10.1089/ars.2013.5566
Tetrahydrobiopterin in cardiovascular health and disease
Abstract
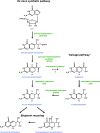
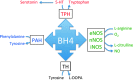
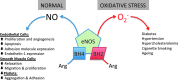
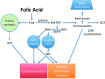
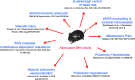
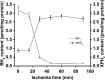
Tetrahydrobiopterin (BH4) functions as a cofactor for several important enzyme systems, and considerable evidence implicates BH4 as a key regulator of endothelial nitric oxide synthase (eNOS) in the setting of cardiovascular health and disease. BH4 bioavailability is determined by a balance of enzymatic de novo synthesis and recycling, versus degradation in the setting of oxidative stress. Augmenting vascular BH4 levels by pharmacological supplementation has been shown in experimental studies to enhance NO bioavailability. However, it has become more apparent that the role of BH4 in other enzymatic pathways, including other NOS isoforms and the aromatic amino acid hydroxylases, may have a bearing on important aspects of vascular homeostasis, inflammation, and cardiac function. This article reviews the role of BH4 in cardiovascular development and homeostasis, as well as in pathophysiological processes such as endothelial and vascular dysfunction, atherosclerosis, inflammation, and cardiac hypertrophy. We discuss the therapeutic potential of BH4 in cardiovascular disease states and attempt to address how this modulator of intracellular NO-redox balance may ultimately provide a powerful new treatment for many cardiovascular diseases.
Figures



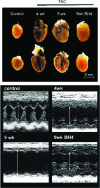
References
-
- Adlam D, Bendall JK, De Bono JP, Alp NJ, Khoo J, Nicoli T, Yokoyama M, Kawashima S, and Channon KM. Relationships between nitric oxide-mediated endothelial function, eNOS coupling and blood pressure revealed by eNOS-GTP cyclohydrolase 1 double transgenic mice. Exp Physiol 92: 119–126, 2007 - PubMed
-
- Ali ZA, Alp N, Tatham AL, Greaves DR, and Channon KM. Increased endothelial tetrahydrobiopterin synthesis reduces vein graft atherosclerosis in ApoE-knockout mice.Circulation 112(17)II, 440, 2005
-
- Ali ZA, Bursill CA, Douglas G, McNeill E, Papaspyridonos M, Tatham AL, Bendall JK, Akhtar AM, Alp NJ, Greaves DR, and Channon KM. CCR2-mediated antiinflammatory effects of endothelial tetrahydrobiopterin inhibit vascular injury-induced accelerated atherosclerosis. Circulation 118: S71–S77, 2008 - PubMed
-
- Alp NJ, McAteer MA, Khoo J, Choudhury RP, and Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 24: 445–450, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
