Efficient large-scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones
- PMID: 24294908
- PMCID: PMC4054944
- DOI: 10.1186/scrt356
Efficient large-scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones
Abstract
Introduction: Embryonic stem (ES) cells are considered a potentially advantageous source of hepatocytes for both transplantation and the development of bioartificial livers. However, the efficient large-scale generation of functional hepatocytes from ES cells remains a major challenge, especially for those methods compatible with clinical applications.
Methods: In this study, we investigated whether a large number of functional hepatocytes can be differentiated from mouse ES (mES) cells using a simulated microgravity bioreactor. mES cells were cultured in a rotating bioreactor in the presence of exogenous growth factors and hormones to form embryoid bodies (EBs), which then differentiated into hepatocytes.
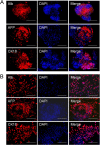
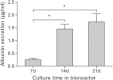
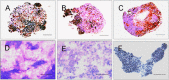
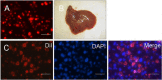
Results: During the rotating culture, most of the EB-derived cells gradually showed the histologic characteristics of normal hepatocytes. More specifically, the expression of hepatic genes and proteins was detected at a higher level in the differentiated cells from the bioreactor culture than in cells from a static culture. On further growing, the EBs on tissue-culture plates, most of the EB-derived cells were found to display the morphologic features of hepatocytes, as well as albumin synthesis. In addition, the EB-derived cells grown in the rotating bioreactor exhibited higher levels of liver-specific functions, such as glycogen storage, cytochrome P450 activity, low-density lipoprotein, and indocyanine green uptake, than did differentiated cells grown in static culture. When the EB-derived cells from day-14 EBs and the cells’ culture supernatant were injected into nude mice, the transplanted cells were engrafted into the recipient livers.
Conclusions: Large quantities of high-quality hepatocytes can be generated from mES cells in a rotating bioreactor via EB formation. This system may be useful in the large-scale generation of hepatocytes for both cell transplantation and the development of bioartificial livers.
Figures





Comment in
-
Large-scale generation of differentiated cells to achieve regenerative medicine.Stem Cell Res Ther. 2014 Jan 20;5(1):10. doi: 10.1186/scrt399. Stem Cell Res Ther. 2014. PMID: 24444304 Free PMC article.
Similar articles
-
Rotating microgravity-bioreactor cultivation enhances the hepatic differentiation of mouse embryonic stem cells on biodegradable polymer scaffolds.Tissue Eng Part A. 2012 Nov;18(21-22):2376-85. doi: 10.1089/ten.TEA.2012.0097. Epub 2012 Sep 24. Tissue Eng Part A. 2012. PMID: 22712633
-
Generation of functional hepatocytes from mouse germ line cell-derived pluripotent stem cells in vitro.Stem Cells Dev. 2010 Aug;19(8):1183-94. doi: 10.1089/scd.2009.0496. Stem Cells Dev. 2010. PMID: 20331356
-
Production of mouse embryoid bodies with hepatic differentiation potential by stirred tank bioreactor.Biosci Biotechnol Biochem. 2007 Mar;71(3):728-34. doi: 10.1271/bbb.60568. Epub 2007 Mar 7. Biosci Biotechnol Biochem. 2007. PMID: 17341832
-
Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells.J Biosci Bioeng. 2007 May;103(5):389-98. doi: 10.1263/jbb.103.389. J Biosci Bioeng. 2007. PMID: 17609152 Review.
-
Bioreactor Technologies for Enhanced Organoid Culture.Int J Mol Sci. 2023 Jul 13;24(14):11427. doi: 10.3390/ijms241411427. Int J Mol Sci. 2023. PMID: 37511186 Free PMC article. Review.
Cited by
-
Effect of microgravity on proliferation and differentiation of embryonic stem cells in an automated culturing system during the TZ-1 space mission.Cell Prolif. 2018 Oct;51(5):e12466. doi: 10.1111/cpr.12466. Epub 2018 Jul 12. Cell Prolif. 2018. PMID: 29999554 Free PMC article.
-
Conserved mechanisms of self-renewal and pluripotency in mouse and human ESCs regulated by simulated microgravity using a 3D clinostat.Cell Death Discov. 2024 Feb 9;10(1):68. doi: 10.1038/s41420-024-01846-2. Cell Death Discov. 2024. PMID: 38336777 Free PMC article.
-
In vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regeneration.Protein Cell. 2015 Aug;6(8):562-74. doi: 10.1007/s13238-015-0180-2. Epub 2015 Jun 19. Protein Cell. 2015. PMID: 26088193 Free PMC article. Review.
-
Space microgravity improves proliferation of human iPSC-derived cardiomyocytes.Stem Cell Reports. 2022 Oct 11;17(10):2272-2285. doi: 10.1016/j.stemcr.2022.08.007. Epub 2022 Sep 8. Stem Cell Reports. 2022. PMID: 36084640 Free PMC article.
-
Large-scale generation of differentiated cells to achieve regenerative medicine.Stem Cell Res Ther. 2014 Jan 20;5(1):10. doi: 10.1186/scrt399. Stem Cell Res Ther. 2014. PMID: 24444304 Free PMC article.
References
-
- Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS 2nd, Lerut J, Nyberg SL, Salizzoni M, Fagan EA, de Hemptinne B, Broelsch CE, Muraca M, Salmeron JM, Rabkin JM, Metselaar HJ, Pratt D, De La Mata M, McChesney LP, Everson GT, Lavin PT, Stevens AC, Pitkin Z, Solomon BA. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;4:660–667. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667–670. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

