NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia
- PMID: 24294978
- PMCID: PMC4086030
- DOI: 10.1089/ars.2012.5129
NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia
Abstract
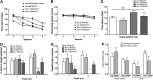
Aims: Chronic cerebral hypoperfusion (CCH) is a common pathological factor that contributes to neurodegenerative diseases such as vascular dementia (VaD). Although oxidative stress has been strongly implicated in the pathogenesis of VaD, the molecular mechanism underlying the selective vulnerability of hippocampal neurons to oxidative damage remains unknown. We assessed whether the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) complex, a specialized superoxide generation system, plays a role in VaD by permanent ligation of bilateral common carotid arteries in rats.
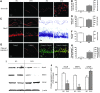
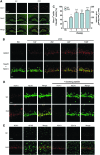
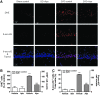
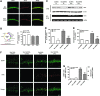
Results: Male Wistar rats (10 weeks of age) were subjected to bilateral occlusion of the common carotid arteries (two-vessel occlusion [2VO]). Nox1 expression gradually increased in hippocampal neurons, starting at 1 week after 2VO and for approximately 15 weeks after 2VO. The levels of superoxide, DNA oxidation, and neuronal death in the CA1 subfield of the hippocampus, as well as consequential cognitive impairment, were increased in 2VO rats. Both inhibition of Nox by apocynin, a putative Nox inhibitor, and adeno-associated virus-mediated Nox1 knockdown significantly reduced 2VO-induced reactive oxygen species generation, oxidative DNA damage, hippocampal neuronal degeneration, and cognitive impairment.
Innovation and conclusion: We provided evidence that neuronal Nox1 is activated in the hippocampus under CCH, causing oxidative stress and consequential hippocampal neuronal death and cognitive impairment. This evidence implies that Nox1-mediated oxidative stress plays an important role in neuronal cell death and cognitive dysfunction in VaD. Nox1 may serve as a potential therapeutic target for VaD.
Figures





References
-
- Aggarwal NT. and Decarli C. Vascular dementia: emerging trends. Semin Neurol 27: 66–77, 2007 - PubMed
-
- Aliev G, Smith MA, Obrenovich ME, de la Torre JC, and Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res 5: 491–504, 2003 - PubMed
-
- Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C, and Butterfield DA. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J Alzheimers Dis 25: 623–633, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
