Investigation of LKB1 Ser431 phosphorylation and Cys433 farnesylation using mouse knockin analysis reveals an unexpected role of prenylation in regulating AMPK activity
- PMID: 24295069
- PMCID: PMC3898322
- DOI: 10.1042/BJ20131324
Investigation of LKB1 Ser431 phosphorylation and Cys433 farnesylation using mouse knockin analysis reveals an unexpected role of prenylation in regulating AMPK activity
Abstract
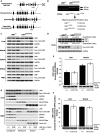
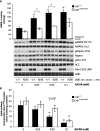
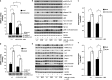
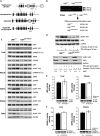
The LKB1 tumour suppressor protein kinase functions to activate two isoforms of AMPK (AMP-activated protein kinase) and 12 members of the AMPK-related family of protein kinases. The highly conserved C-terminal residues of LKB1 are phosphorylated (Ser431) by PKA (cAMP-dependent protein kinase) and RSK (ribosomal S6 kinase) and farnesylated (Cys433) within a CAAX motif. To better define the role that these post-translational modifications play, we created homozygous LKB1S431A/S431A and LKB1C433S/C433S knockin mice. These animals were viable, fertile and displayed no overt phenotypes. Employing a farnesylation-specific monoclonal antibody that we generated, we established by immunoprecipitation that the vast majority, if not all, of the endogenous LKB1 is prenylated. Levels of LKB1 localized at the membrane of the liver of LKB1C433S/C433S mice and their fibroblasts were reduced substantially compared with the wild-type mice, confirming that farnesylation plays a role in mediating membrane association. Although AMPK was activated normally in the LKB1S431A/S431A animals, we unexpectedly observed in all of the examined tissues and cells taken from LKB1C433S/C433S mice that the basal, as well as that induced by the AMP-mimetic AICAR (5-amino-4-imidazolecarboxamide riboside), AMPK activation, phenformin and muscle contraction were significantly blunted. This resulted in a reduced ability of AICAR to inhibit lipid synthesis in primary hepatocytes isolated from LKB1C433S/C433S mice. The activity of several of the AMPK-related kinases analysed [BRSK1 (BR serine/threonine kinase 1), BRSK2, NUAK1 (NUAK family, SNF1-like kinase 1), SIK3 (salt-inducible kinase 3) and MARK4 (MAP/microtubule affinity-regulating kinase 4)] was not affected in tissues derived from LKB1S431A/S431A or LKB1C433S/C433S mice. Our observations reveal for the first time that farnesylation of LKB1 is required for the activation of AMPK. Previous reports have indicated that a pool of AMPK is localized at the plasma membrane as a result of myristoylation of its regulatory AMPKβ subunit. This raises the possibility that LKB1 farnesylation and myristoylation of AMPKβ might promote the interaction and co-localization of these enzymes on a two-dimensional membrane surface and thereby promote efficient activation of AMPK.
Figures



References
-
- Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Hoglund P., et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. - PubMed
-
- Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz–Jeghers syndrome. Oncogene. 2007;26:7825–7832. - PubMed
-
- Ji H., Ramsey M. R., Hayes D. N., Fan C., McNamara K., Kozlowski P., Torrice C., Wu M. C., Shimamura T., Perera S. A., et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. - PubMed
-
- Sanchez-Cespedes M., Parrella P., Esteller M., Nomoto S., Trink B., Engles J. M., Westra W. H., Herman J. G., Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

