Central role of RET in thyroid cancer
- PMID: 24296167
- PMCID: PMC3839608
- DOI: 10.1101/cshperspect.a009233
Central role of RET in thyroid cancer
Abstract
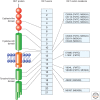
RET (rearranged during transfection) is a receptor tyrosine kinase involved in the development of neural crest derived cell lineages, kidney, and male germ cells. Different human cancers, including papillary and medullary thyroid carcinomas, lung adenocarcinomas, and myeloproliferative disorders display gain-of-function mutations in RET. Accordingly, RET protein has become a promising molecular target for cancer treatment.
Figures



References
-
- Airaksinen MS, Saarma M 2002. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394 - PubMed
-
- Alberti L, Borrello MG, Ghizzoni S, Torriti F, Rizzetti MG, Pierotti MA 1998. Grb2 binding to the different isoforms of Ret tyrosine kinase. Oncogene 17: 1079–1087 - PubMed
-
- Ameziane-El-Hassani R, Boufraqech M, Lagente-Chevallier O, Weyemi U, Talbot M, Métivier D, Courtin F, Bidart JM, El Mzibri M, Schlumberger M, et al. 2010. Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res 70: 4123–4132 - PubMed
-
- Anders J, Kjar S, Ibáñez CF 2001. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem 276: 35808–35817 - PubMed
-
- Arighi E, Popsueva A, Degl’Innocenti D, Borrello MG, Carniti C, Perälä NM, Pierotti MA, Sariola H 2004. Biological effects of the dual phenotypic Janus mutation of ret cosegregating with both multiple endocrine neoplasia type 2 and Hirschsprung’s disease. Mol Endocrinol 18: 1004–1017 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
