Endocytosis and cancer
- PMID: 24296170
- PMCID: PMC3839607
- DOI: 10.1101/cshperspect.a016949
Endocytosis and cancer
Abstract
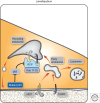
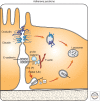
Endocytosis entails selective packaging of cell-surface proteins, such as receptors for cytokines and adhesion components, in cytoplasmic vesicles (endosomes). The series of sorting events that determines the fate of internalized proteins, either degradation in lysosomes or recycling back to the plasma membrane, relies on intrinsic sequence motifs, posttranslational modifications (e.g., phosphorylation and ubiquitination), and transient assemblies of both Rab GTPases and phosphoinositide-binding proteins. This multicomponent process is enhanced and skewed in cancer cells; we review mechanisms enabling both major drivers of cancer, p53 and Ras, to bias recycling of integrins and receptor tyrosine kinases (RTKs). Likewise, cadherins and other junctional proteins of cancer cells are constantly removed from the cell surface, thereby disrupting tissue polarity and instigating motile phenotypes. Mutant forms of RTKs able to evade Cbl-mediated ubiquitination, along with overexpression of the wild-type forms and a variety of defective feedback regulatory loops, are frequently detected in tumors. Finally, we describe pharmacological attempts to harness the peculiar endocytic system of cancer, in favor of effective patient treatment.
Figures



References
-
- Anastasi S, Fiorentino L, Fiorini M, Fraioli R, Sala G, Castellani L, Alema S, Alimandi M, Segatto O 2003. Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene 22: 4221–4234 - PubMed
-
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK 2006. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8: 1235–1245 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
