SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK
- PMID: 24296486
- PMCID: PMC3868726
- DOI: 10.18632/aging.100616
SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK
Abstract
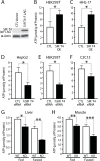
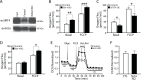
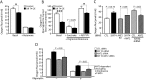
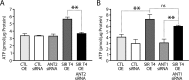
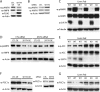
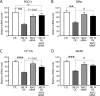
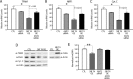
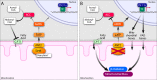
Efficient coupling of cellular energy production to metabolic demand is crucial to maintain organismal homeostasis. Here, we report that the mitochondrial Sirtuin Sirt4 regulates mitochondrial ATP homeostasis. We find that Sirt4 affects mitochondrial uncoupling via the adenine nucleotide translocator 2 (ANT2). Loss of Sirt4 expression leads to decreased cellular ATP levelsin vitro and in vivo while Sirt4 overexpression is associated with increased ATP levels. Further, we provide evidence that lack of Sirt4 activates a retrograde signaling response from the mitochondria to the nucleus that includes AMPK, PGC1α, key regulators of β-oxidation such as Acetyl-CoA carboxylase, and components of the mitochondrial respiratory machinery. This study highlights the ability of Sirt4 to regulate ATP levels via ANT2 and a feedback loop involving AMPK.
Conflict of interest statement
Eric Verdin is member of the advisory board of Sirtris/GSK.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

