Identification of IFN-γ-producing innate B cells
- PMID: 24296781
- PMCID: PMC3915900
- DOI: 10.1038/cr.2013.155
Identification of IFN-γ-producing innate B cells
Abstract
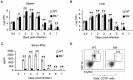
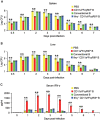
Although B cells play important roles in the humoral immune response and the regulation of adaptive immunity, B cell subpopulations with unique phenotypes, particularly those with non-classical immune functions, should be further investigated. By challenging mice with Listeria monocytogenes, Escherichia coli, vesicular stomatitis virus and Toll-like receptor ligands, we identified an inducible CD11a(hi)FcγRIII(hi) B cell subpopulation that is significantly expanded and produces high levels of IFN-γ during the early stage of the immune response. This subpopulation of B cells can promote macrophage activation via generating IFN-γ, thereby facilitating the innate immune response against intracellular bacterial infection. As this new subpopulation is of B cell origin and exhibits the phenotypic characteristics of B cells, we designated these cells as IFN-γ-producing innate B cells. Dendritic cells were essential for the inducible generation of these innate B cells from the follicular B cells via CD40L-CD40 ligation. Increased Bruton's tyrosine kinase activation was found to be responsible for the increased activation of non-canonical NF-κB pathway in these innate B cells after CD40 ligation, with the consequent induction of additional IFN-γ production. The identification of this new population of innate B cells may contribute to a better understanding of B cell functions in anti-infection immune responses and immune regulation.
Figures






Comment in
-
Innate IFNγ-producing B cells.Cell Res. 2014 Feb;24(2):135-6. doi: 10.1038/cr.2013.163. Epub 2013 Dec 17. Cell Res. 2014. PMID: 24343577 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

