Measuring single cell mass, volume, and density with dual suspended microchannel resonators
- PMID: 24296901
- PMCID: PMC3895367
- DOI: 10.1039/c3lc51022k
Measuring single cell mass, volume, and density with dual suspended microchannel resonators
Abstract
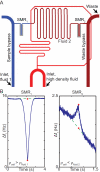
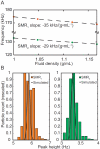
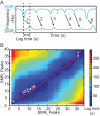
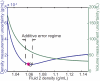
Cell size, measured as either volume or mass, is a fundamental indicator of cell state. Far more tightly regulated than size is density, the ratio between mass and volume, which can be used to distinguish between cell populations even when volume and mass appear to remain constant. Here we expand upon a previous method for measuring cell density involving a suspended microchannel resonator (SMR). We introduce a new device, the dual SMR, as a high-precision instrument for measuring single-cell mass, volume, and density using two resonators connected by a serpentine fluidic channel. The dual SMR designs considered herein demonstrate the critical role of channel geometry in ensuring proper mixing and damping of pressure fluctuations in microfluidic systems designed for precision measurement. We use the dual SMR to compare the physical properties of two well-known cancer cell lines: human lung cancer cell H1650 and mouse lymphoblastic leukemia cell line L1210.
Figures

References
-
- Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Vol. 27. Garland Publishing, Inc; New York: 1998.
-
- Park J-M, Lee J-Y, Lee J-G, Jeong H, Oh J-M, Kim YJ, Park D, Kim MS, Lee HJ, Oh JH, Lee SS, Lee W-Y, Huh N. Analytical chemistry. 2012;84:7400–7. - PubMed
-
- Friedman SL, Roll FJ. Analytical biochemistry. 1987;161:207–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

