Membrane and chaperone recognition by the major translocator protein PopB of the type III secretion system of Pseudomonas aeruginosa
- PMID: 24297169
- PMCID: PMC3916559
- DOI: 10.1074/jbc.M113.517920
Membrane and chaperone recognition by the major translocator protein PopB of the type III secretion system of Pseudomonas aeruginosa
Abstract
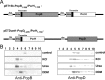
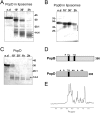
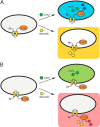
The type III secretion system is a widespread apparatus used by pathogenic bacteria to inject effectors directly into the cytoplasm of eukaryotic cells. A key component of this highly conserved system is the translocon, a pore formed in the host membrane that is essential for toxins to bypass this last physical barrier. In Pseudomonas aeruginosa the translocon is composed of PopB and PopD, both of which before secretion are stabilized within the bacterial cytoplasm by a common chaperone, PcrH. In this work we characterize PopB, the major translocator, in both membrane-associated and PcrH-bound forms. By combining sucrose gradient centrifugation experiments, limited proteolysis, one-dimensional NMR, and β-lactamase reporter assays on eukaryotic cells, we show that PopB is stably inserted into bilayers with its flexible N-terminal domain and C-terminal tail exposed to the outside. In addition, we also report the crystal structure of the complex between PcrH and an N-terminal region of PopB (residues 51-59), which reveals that PopB lies within the concave face of PcrH, employing mostly backbone residues for contact. PcrH is thus the first chaperone whose structure has been solved in complex with both type III secretion systems translocators, revealing that both molecules employ the same surface for binding and excluding the possibility of formation of a ternary complex. The characterization of the major type III secretion system translocon component in both membrane-bound and chaperone-bound forms is a key step for the eventual development of antibacterials that block translocon assembly.
Keywords: Crystal Structure; Host-Pathogen Interactions; Membrane Proteins; Pseudomonas aeruginosa; Type III Secretion System.
Figures




Similar articles
-
Binding mode analysis of a major T3SS translocator protein PopB with its chaperone PcrH from Pseudomonas aeruginosa.Proteins. 2014 Dec;82(12):3273-85. doi: 10.1002/prot.24666. Epub 2014 Oct 21. Proteins. 2014. PMID: 25116453
-
Structural basis of chaperone recognition of type III secretion system minor translocator proteins.J Biol Chem. 2010 Jul 23;285(30):23224-32. doi: 10.1074/jbc.M110.111278. Epub 2010 Apr 12. J Biol Chem. 2010. PMID: 20385547 Free PMC article.
-
Type III secretion system translocator has a molten globule conformation both in its free and chaperone-bound forms.FEBS J. 2007 Jul;274(14):3601-3610. doi: 10.1111/j.1742-4658.2007.05893.x. Epub 2007 Jun 18. FEBS J. 2007. PMID: 17578515
-
Structure and biophysics of type III secretion in bacteria.Biochemistry. 2013 Apr 16;52(15):2508-17. doi: 10.1021/bi400160a. Epub 2013 Apr 5. Biochemistry. 2013. PMID: 23521714 Free PMC article. Review.
-
Composition, function, and regulation of T6SS in Pseudomonas aeruginosa.Microbiol Res. 2015 Mar;172:19-25. doi: 10.1016/j.micres.2015.01.004. Epub 2015 Jan 7. Microbiol Res. 2015. PMID: 25721475 Review.
Cited by
-
Topological analysis of type 3 secretion translocons in native membranes.Methods Enzymol. 2021;649:397-429. doi: 10.1016/bs.mie.2021.01.036. Epub 2021 Feb 26. Methods Enzymol. 2021. PMID: 33712194 Free PMC article.
-
PopB-PcrV Interactions Are Essential for Pore Formation in the Pseudomonas aeruginosa Type III Secretion System Translocon.mBio. 2022 Oct 26;13(5):e0238122. doi: 10.1128/mbio.02381-22. Epub 2022 Sep 26. mBio. 2022. PMID: 36154276 Free PMC article.
-
A Pseudomonas aeruginosa-Derived Particulate Vaccine Protects against P. aeruginosa Infection.Vaccines (Basel). 2021 Jul 20;9(7):803. doi: 10.3390/vaccines9070803. Vaccines (Basel). 2021. PMID: 34358220 Free PMC article.
-
Direct interaction of a chaperone-bound type III secretion substrate with the export gate.Nat Commun. 2022 Jun 2;13(1):2858. doi: 10.1038/s41467-022-30487-1. Nat Commun. 2022. PMID: 35654781 Free PMC article.
-
Acylation of the Type 3 Secretion System Translocon Using a Dedicated Acyl Carrier Protein.PLoS Genet. 2017 Jan 13;13(1):e1006556. doi: 10.1371/journal.pgen.1006556. eCollection 2017 Jan. PLoS Genet. 2017. PMID: 28085879 Free PMC article.
References
-
- Cornelis G. R. (2006) The type III secretion injectisome. Nat. Rev. Microbiol. 4, 811–825 - PubMed
-
- Blocker A., Jouihri N., Larquet E., Gounon P., Ebel F., Parsot C., Sansonetti P., Allaoui A. (2001) Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39, 652–663 - PubMed
-
- Zarivach R., Vuckovic M., Deng W., Finlay B. B., Strynadka N. C. (2007) Structural analysis of a prototypical ATPase from the type III secretion system. Nat. Struct. Mol. Biol. 14, 131–137 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

