Understanding how the complex molecular architecture of mannan-degrading hydrolases contributes to plant cell wall degradation
- PMID: 24297170
- PMCID: PMC3900950
- DOI: 10.1074/jbc.M113.527770
Understanding how the complex molecular architecture of mannan-degrading hydrolases contributes to plant cell wall degradation
Abstract
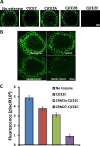
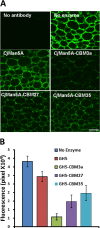
Microbial degradation of plant cell walls is a central component of the carbon cycle and is of increasing importance in environmentally significant industries. Plant cell wall-degrading enzymes have a complex molecular architecture consisting of catalytic modules and, frequently, multiple non-catalytic carbohydrate binding modules (CBMs). It is currently unclear whether the specificities of the CBMs or the topology of the catalytic modules are the primary drivers for the specificity of these enzymes against plant cell walls. Here, we have evaluated the relationship between CBM specificity and their capacity to enhance the activity of GH5 and GH26 mannanases and CE2 esterases against intact plant cell walls. The data show that cellulose and mannan binding CBMs have the greatest impact on the removal of mannan from tobacco and Physcomitrella cell walls, respectively. Although the action of the GH5 mannanase was independent of the context of mannan in tobacco cell walls, a significant proportion of the polysaccharide was inaccessible to the GH26 enzyme. The recalcitrant mannan, however, was fully accessible to the GH26 mannanase appended to a cellulose binding CBM. Although CE2 esterases display similar specificities against acetylated substrates in vitro, only CjCE2C was active against acetylated mannan in Physcomitrella. Appending a mannan binding CBM27 to CjCE2C potentiated its activity against Physcomitrella walls, whereas a xylan binding CBM reduced the capacity of esterases to deacetylate xylan in tobacco walls. This work provides insight into the biological significance for the complex array of hydrolytic enzymes expressed by plant cell wall-degrading microorganisms.
Keywords: Carbohydrate-binding Protein; Glycoside Hydrolases; Microscopic Imaging; Plant Cell Wall; Polysaccharide.
Figures




Similar articles
-
Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects.Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15293-8. doi: 10.1073/pnas.1005732107. Epub 2010 Aug 9. Proc Natl Acad Sci U S A. 2010. PMID: 20696902 Free PMC article.
-
The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation.Biochem J. 2003 May 1;371(Pt 3):1027-43. doi: 10.1042/BJ20021860. Biochem J. 2003. PMID: 12523937 Free PMC article.
-
X4 modules represent a new family of carbohydrate-binding modules that display novel properties.J Biol Chem. 2004 May 28;279(22):22953-63. doi: 10.1074/jbc.M313317200. Epub 2004 Mar 5. J Biol Chem. 2004. PMID: 15004012
-
Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules.Curr Opin Struct Biol. 2013 Oct;23(5):669-77. doi: 10.1016/j.sbi.2013.05.005. Epub 2013 Jun 13. Curr Opin Struct Biol. 2013. PMID: 23769966 Review.
-
Towards enzymatic breakdown of complex plant xylan structures: State of the art.Biotechnol Adv. 2016 Nov 15;34(7):1260-1274. doi: 10.1016/j.biotechadv.2016.09.001. Epub 2016 Sep 9. Biotechnol Adv. 2016. PMID: 27620948 Review.
Cited by
-
Comprehensive functional characterization of the glycoside hydrolase family 3 enzymes from Cellvibrio japonicus reveals unique metabolic roles in biomass saccharification.Environ Microbiol. 2017 Dec;19(12):5025-5039. doi: 10.1111/1462-2920.13959. Epub 2017 Dec 7. Environ Microbiol. 2017. PMID: 29052930 Free PMC article.
-
A Comparative Study of Sample Preparation for Staining and Immunodetection of Plant Cell Walls by Light Microscopy.Front Plant Sci. 2017 Aug 29;8:1505. doi: 10.3389/fpls.2017.01505. eCollection 2017. Front Plant Sci. 2017. PMID: 28900439 Free PMC article.
-
The Contribution of Non-catalytic Carbohydrate Binding Modules to the Activity of Lytic Polysaccharide Monooxygenases.J Biol Chem. 2016 Apr 1;291(14):7439-49. doi: 10.1074/jbc.M115.702365. Epub 2016 Jan 22. J Biol Chem. 2016. PMID: 26801613 Free PMC article.
-
CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed.Plant Physiol. 2014 Apr;164(4):1842-56. doi: 10.1104/pp.114.236596. Epub 2014 Feb 25. Plant Physiol. 2014. PMID: 24569843 Free PMC article.
-
Determination of glycoside hydrolase specificities during hydrolysis of plant cell walls using glycome profiling.Biotechnol Biofuels. 2017 Feb 2;10:31. doi: 10.1186/s13068-017-0703-6. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28184246 Free PMC article.
References
-
- Himmel M. E., Bayer E. A. (2009) Lignocellulose conversion to biofuels. Current challenges, global perspectives. Curr. Opin. Biotechnol. 20, 316–317 - PubMed
-
- Himmel M. E., Ding S. Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., Foust T. D. (2007) Biomass recalcitrance. Engineering plants and enzymes for biofuels production. Science 315, 804–807 - PubMed
-
- Brett C. T., Waldren K. (1996) Physiology and Biochemistry of Plant Cell Walls, 2nd Ed., Chapman and Hall, London
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

