Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo
- PMID: 24297173
- PMCID: PMC3894339
- DOI: 10.1074/jbc.M113.507228
Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo
Abstract
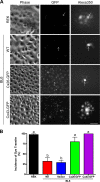
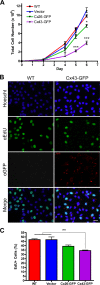
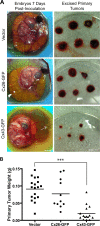
Connexins (Cx) have been identified as tumor suppressors or enhancers, a distinction that appears to be dependent on the type and stage of disease. However, the role of connexins in melanoma tumorigenesis and their status during cancer onset and progression remain controversial and unclear. Here, we show that the aggressive B16-BL6 mouse melanoma cell line expresses low basal levels of Cx26 and Cx43, rendering them gap junctional intercellular communication-deficient as elucidated by immunofluorescence, Western blotting, and dye transfer studies. Following ectopic expression of green fluorescent protein-tagged Cx26 and Cx43 in these connexin-deficient melanomas, punctate gap junction-like plaques were evident at sites of cell-cell apposition, and the incidence of dye transfer was significantly increased similar to connexin-rich keratinocytes. We found that the expression of Cx43, but not Cx26, significantly reduced cellular proliferation and anchorage-independent growth from control melanomas, whereas migration was unaffected. Additionally, melanomas expressing Cx43 displayed significantly reduced growth within the in situ-like microenvironment of keratinocytes, despite a lack of heterocellular gap junctional intercellular communication between the two cell types. Furthermore, when grown in vivo in the chicken chorioallantoic membrane, primary tumors derived from Cx43-expressing melanomas were significantly smaller than controls, whereas Cx26-expressing melanomas produced tumors similar to controls. Collectively, these results suggest that Cx43, and not Cx26, can act as a tumor suppressor during melanoma tumorigenesis.
Keywords: Connexin; Gap Junctions; Keratinocytes; Melanoma; Tumor Suppressor Gene.
Figures





References
-
- Söhl G., Willecke K. (2003) An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 10, 173–180 - PubMed
-
- Richard G. (2000) Connexins. A connection with the skin. Exp. Dermatol. 9, 77–96 - PubMed
-
- Hsu M., Andl T., Li G., Meinkoth J. L., Herlyn M. (2000) Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J. Cell Sci. 113, 1535–1542 - PubMed
-
- Masuda M., Usami S., Yamazaki K., Takumi Y., Shinkawa H., Kurashima K., Kunihiro T., Kanzaki J. (2001) Connexin 26 distribution in gap junctions between melanocytes in the human vestibular dark cell area. Anat. Rec. 262, 137–146 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

