Mitochondria contribute to NADPH generation in mouse rod photoreceptors
- PMID: 24297174
- PMCID: PMC3894333
- DOI: 10.1074/jbc.M113.511295
Mitochondria contribute to NADPH generation in mouse rod photoreceptors
Abstract
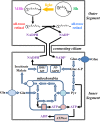
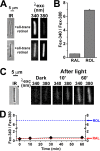
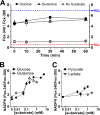
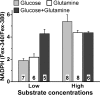
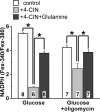
NADPH is the primary source of reducing equivalents in the cytosol. Its major source is considered to be the pentose phosphate pathway, but cytosolic NADP(+)-dependent dehydrogenases using intermediates of mitochondrial pathways for substrates have been known to contribute. Photoreceptors, a nonproliferating cell type, provide a unique model for measuring the functional utilization of NADPH at the single cell level. In these cells, NADPH availability can be monitored from the reduction of the all-trans-retinal generated by light to all-trans-retinol using single cell fluorescence imaging. We have used mouse rod photoreceptors to investigate the generation of NADPH by different metabolic pathways. In the absence of extracellular metabolic substrates, NADPH generation was severely compromised. Extracellular glutamine supported NADPH generation to levels comparable to those of glucose, but pyruvate and lactate were relatively ineffective. At low extracellular substrate concentrations, partial inhibition of ATP synthesis lowered, whereas suppression of ATP consumption augmented NADPH availability. Blocking pyruvate transport into mitochondria decreased NADPH availability, and addition of glutamine restored it. Our findings demonstrate that in a nonproliferating cell type, mitochondria-linked pathways can generate substantial amounts of NADPH and do so even when the pentose phosphate pathway is operational. Competing demands for ATP and NADPH at low metabolic substrate concentrations indicate a vulnerability to nutrient shortages. By supporting substantial NADPH generation, mitochondria provide alternative metabolic pathways that may support cell function and maintain viability under transient nutrient shortages. Such pathways may play an important role in protecting against retinal degeneration.
Keywords: Fluorescence; Metabolism; Mitochondria; NADPH; Photoreceptors; Retinoid.
Figures




References
-
- Nelson D. L., Cox M. M. (2000) Lehninger Principles of Biochemistry, pp. 558–562, Worth, New York
-
- Lunt S. Y., Vander Heiden M. G. (2011) Aerobic glycolysis. Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 - PubMed
-
- DeBerardinis R. J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C. B. (2007) Beyond aerobic glycolysis. Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 104, 19345–19350 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

