Strategies to improve retention in randomised trials
- PMID: 24297482
- PMCID: PMC4470347
- DOI: 10.1002/14651858.MR000032.pub2
Strategies to improve retention in randomised trials
Abstract
Background: Loss to follow-up from randomised trials can introduce bias and reduce study power, affecting the generalisability, validity and reliability of results. Many strategies are used to reduce loss to follow-up and improve retention but few have been formally evaluated.
Objectives: To quantify the effect of strategies to improve retention on the proportion of participants retained in randomised trials and to investigate if the effect varied by trial strategy and trial setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PreMEDLINE, EMBASE, PsycINFO, DARE, CINAHL, Campbell Collaboration's Social, Psychological, Educational and Criminological Trials Register, and ERIC. We handsearched conference proceedings and publication reference lists for eligible retention trials. We also surveyed all UK Clinical Trials Units to identify further studies.
Selection criteria: We included eligible retention trials of randomised or quasi-randomised evaluations of strategies to increase retention that were embedded in 'host' randomised trials from all disease areas and healthcare settings. We excluded studies aiming to increase treatment compliance.
Data collection and analysis: We contacted authors to supplement or confirm data that we had extracted. For retention trials, we recorded data on the method of randomisation, type of strategy evaluated, comparator, primary outcome, planned sample size, numbers randomised and numbers retained. We used risk ratios (RR) to evaluate the effectiveness of the addition of strategies to improve retention. We assessed heterogeneity between trials using the Chi(2) and I(2) statistics. For main trials that hosted retention trials, we extracted data on disease area, intervention, population, healthcare setting, sequence generation and allocation concealment.
Main results: We identified 38 eligible retention trials. Included trials evaluated six broad types of strategies to improve retention. These were incentives, communication strategies, new questionnaire format, participant case management, behavioural and methodological interventions. For 34 of the included trials, retention was response to postal and electronic questionnaires with or without medical test kits. For four trials, retention was the number of participants remaining in the trial. Included trials were conducted across a spectrum of disease areas, countries, healthcare and community settings. Strategies that improved trial retention were addition of monetary incentives compared with no incentive for return of trial-related postal questionnaires (RR 1.18; 95% CI 1.09 to 1.28, P value < 0.0001), addition of an offer of monetary incentive compared with no offer for return of electronic questionnaires (RR 1.25; 95% CI 1.14 to 1.38, P value < 0.00001) and an offer of a GBP20 voucher compared with GBP10 for return of postal questionnaires and biomedical test kits (RR 1.12; 95% CI 1.04 to 1.22, P value < 0.005). The evidence that shorter questionnaires are better than longer questionnaires was unclear (RR 1.04; 95% CI 1.00 to 1.08, P value = 0.07) and the evidence for questionnaires relevant to the disease/condition was also unclear (RR 1.07; 95% CI 1.01 to 1.14). Although each was based on the results of a single trial, recorded delivery of questionnaires seemed to be more effective than telephone reminders (RR 2.08; 95% CI 1.11 to 3.87, P value = 0.02) and a 'package' of postal communication strategies with reminder letters appeared to be better than standard procedures (RR 1.43; 95% CI 1.22 to 1.67, P value < 0.0001). An open trial design also appeared more effective than a blind trial design for return of questionnaires in one fracture prevention trial (RR 1.37; 95% CI 1.16 to 1.63, P value = 0.0003).There was no good evidence that the addition of a non-monetary incentive, an offer of a non-monetary incentive, 'enhanced' letters, letters delivered by priority post, additional reminders, or questionnaire question order either increased or decreased trial questionnaire response/retention. There was also no evidence that a telephone survey was either more or less effective than a monetary incentive and a questionnaire. As our analyses are based on single trials, the effect on questionnaire response of using offers of charity donations, sending reminders to trial sites and when a questionnaire is sent, may need further evaluation. Case management and behavioural strategies used for trial retention may also warrant further evaluation.
Authors' conclusions: Most of the retention trials that we identified evaluated questionnaire response. There were few evaluations of ways to improve participants returning to trial sites for trial follow-up. Monetary incentives and offers of monetary incentives increased postal and electronic questionnaire response. Some other strategies evaluated in single trials looked promising but need further evaluation. Application of the findings of this review would depend on trial setting, population, disease area, data collection and follow-up procedures.
Figures




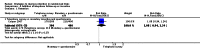

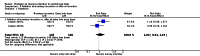
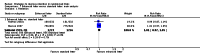









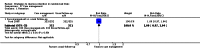

References
-
- Ashby R, Turner G, Cross B, Mitchell N, Torgerson D. A randomized trial of electronic reminders showed a reduction in the time to respond to postal questionnaires. Journal of Clinical Epidemiology. 2011;64:208–12. - PubMed
-
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA, et al. The effects of an open design on trial participant recruitment, compliance and retention - a randomized controlled trial comparison with a blinded, placebo-controlled design. Clinical Trials. 2004;1((6)):490–8. - PubMed
-
- Bailey J. Evaluating the effectiveness of incentives in the "Sexunzipped" trial
-
- Bailey J. Evaluating the effectiveness of incentives in the "Sexunzipped" trial
-
- Bauer JE, Rezaishiraz H, Head K, Cowell J, Bepler G, Aiken M, et al. Obtaining DNA from a geographically dispersed cohort of current and former smokers: use of mail-based mouthwash collection and monetary incentives. Nicotine and Tobacco Research. 2004;6((3)):439–46. - PubMed
References to studies excluded from this review
-
- Arnevik E, Wilberg T, Urnes Ø, Johansen M, Monsen J, Karterud S. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy - a randomized controlled study. European Psychiatry. 2009;24:71–8. - PubMed
-
- Atherton H, Oakeshott P, Aghaizu A, Hay P, Kerry S. Use of an online questionnaire for follow-up of young female students recruited to a randomised controlled trial of chlamydia screening. Journal of Epidemiology and Community Health. 2010;64((580)):e584. - PubMed
-
- Barry MJ, Walker-Corkery E, Chang Y, Tyll LT, Cherkin DC, Fowler FJ. Measurement of overall and disease-specific health status: does the order of questionnaires make a difference. Journal of Health Services Research and Policy. 1996;1((1)):20–7. - PubMed
-
- Bednarek P, Nichols M, Carlson N, Edelman A, Creinin M, Truitt S, et al. Effect of "observed start" vs. traditional "Sunday start" on hormonal contraceptive continuation rates after medical abortion. Contraception. 2008;78:26–30. - PubMed
-
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40–65 years: the S.W.E.A.T. study (Sedentary Women Exercise Adherence Trial) Preventive Medicine. 2003;36:17–29. - PubMed
References to ongoing studies
-
- Randomised Evaluation of NSABP BAHO Compliance Initiatives. Ongoing study Unclear.
-
- A Randomised Controlled Trial of Combined Pre-Contact and Participant Update for Increasing Questionnaire Response Rates in Older Women. 2010. Ongoing study.
Additional references
-
- Anastasi JK, Capili B, Kim G, Chung A. Clinical trial recruitment and retention of a vulnerable population: HIV patients with chronic diarrhea. Gastroenterology Nursing. 2005;28((6)):463–8. - PubMed
-
- Arnow BA, Blasey C, Manber R, Constantino MJ, Markowitz JC, Klein DN, et al. Dropouts versus completers among chronically depressed outpatients. Journal of Affective Disorders. 2007;97((1-3)):197–202. - PubMed
-
- The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. New England Journal of Medicine. 1997;337((22)):1576–84. - PubMed
-
- Boyd N, Cousins M, Lockwood G, Trichler D. Dietary fat and breast cancer risk: the feasibility of a clinical trial of breast cancer prevention. Lipids. 1992;27((10)):821–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

