Randomized, double-blind, placebo-controlled, crossover study of the effects of lisdexamfetamine dimesylate and mixed amphetamine salts on cognition throughout the day in adults with attention-deficit/hyperactivity disorder
- PMID: 24297663
- PMCID: PMC3899471
- DOI: 10.1007/s40261-013-0156-z
Randomized, double-blind, placebo-controlled, crossover study of the effects of lisdexamfetamine dimesylate and mixed amphetamine salts on cognition throughout the day in adults with attention-deficit/hyperactivity disorder
Abstract
Background: Understanding the nature and time course of the pharmacodynamic effects of attention-deficit/hyperactivity disorder (ADHD) medications is useful. The Cognitive Drug Research Computerized Battery of Tests (CDR-CBT) is a 20-min battery of ten standardized, validated neuropsychometric tasks.
Objective: This pilot study examined the sensitivity and responsiveness of the CDR-CBT for assessing cognitive function in adults with ADHD prior to and up to 16 h postdose during treatment with lisdexamfetamine dimesylate (LDX) or mixed amphetamine salts immediate release (MAS-IR; various generics available).
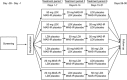
Methods: This was a double-blind three-period crossover study. Participants received LDX 50 mg/day, MAS-IR 20 mg/day, and placebo (~7 a.m.) for 7 days each in randomized order. CDR-CBT was administered on day 1 of period 1 and day 7 of each period at scheduled times between -0.5 (predose) and 16 h postdose. Composite power of attention (PoA) score (sum of simple reaction time, choice reaction time, and digit vigilance speed) was the primary outcome measure. The Conners' Adult ADHD Rating Scales-Self-Report: Short Version (CAARS-S:S) was administered at baseline and on day 1 of period 1, and days 6 and 7 of each treatment period. Tertiary outcomes included CDR-CBT composite continuity of attention scores, its component task scores, cognitive reaction time, and response variability scores. No inferential statistical comparisons were conducted. Safety assessments included adverse events (AEs) and vital signs.
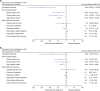
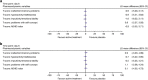
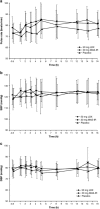
Results: This analysis included 18 participants (mean age 30.8 years); one withdrew because of AEs. Mean pretreatment PoA scores were 1175.9-1361.2 ms, scores commensurate with a normative age of >40 years. Maximum reductions in PoA scores with LDX and MAS-IR occurred at 5 h postdose at day 7 (least squares mean difference [95% CI] of -150.0 [-235.41 to -64.50] and -79.8 [-165.72 to 6.21] ms vs. placebo, respectively). CAARS-S:S scores were unchanged with LDX and MAS-IR (vs. placebo) at all postdose timepoints. Tertiary attention-related CDR-CBT outcomes were sensitive to LDX and MAS-IR (vs. placebo). Treatment-emergent AEs and vital signs were consistent with previous studies in adult ADHD.
Conclusion: In adults with ADHD, PoA scores indicated impaired attention at baseline and response to treatment with LDX and MAS-IR (vs. placebo), demonstrating value for measuring the time course of pharmacologic treatment effects.
Figures

References
-
- Sandra Kooij JJ, Marije Boonstra A, Swinkels SH, et al. Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008;11 (4):445–58. - PubMed
-
- Cubillo A, Halari R, Ecker C, et al. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44(10):629–639. doi: 10.1016/j.jpsychires.2009.11.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

