Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice
- PMID: 24297884
- PMCID: PMC3870707
- DOI: 10.1073/pnas.1318624110
Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice
Abstract
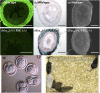
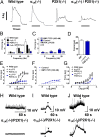
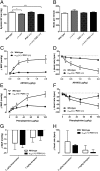
Therapeutic targets for male contraception are associated with numerous problems due to their focus on disrupting spermatogenesis or hormonal mechanisms to produce dysfunctional sperm. Here we describe the dual genetic deletion of α1A-adrenergic G protein-coupled receptors (adrenoceptors) and P2X1-purinoceptor ligand gated ion channels in male mice, thereby blocking sympathetically mediated sperm transport through the vas deferens during the emission phase of ejaculation. This modification produced 100% infertility without effects on sexual behavior or function. Sperm taken from the cauda epididymides of double knockout mice were microscopically normal and motile. Furthermore, double knockout sperm were capable of producing normal offspring following intracytoplasmic sperm injection into wild-type ova and implantation of the fertilized eggs into foster mothers. Blood pressure and baroreflex function was reduced in double knockout mice, but no more than single knockout of α1A-adrenoceptors alone. These results suggest that this autonomic method of male contraception appears free of major physiological and behavioral side effects. In addition, they provide conclusive proof of concept that pharmacological antagonism of the P2X1-purinoceptor and α1A-adrenoceptor provides a safe and effective therapeutic target for a nonhormonal, readily reversible male contraceptive.
Keywords: ATP; noradrenaline; sympathetic neurotransmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bruschini H, Schmidt RA, Tanagho EA. Neurologic control of prostatic secretion in the dog. Invest Urol. 1978;15(4):288–290. - PubMed
-
- Steers WD. Physiology of the vas deferens. World J Urol. 1994;12(5):281–285. - PubMed
-
- Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: Further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100(1):85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

