Optical control of an ion channel gate
- PMID: 24297890
- PMCID: PMC3870725
- DOI: 10.1073/pnas.1318715110
Optical control of an ion channel gate
Abstract
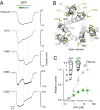
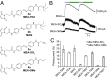
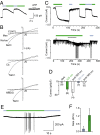
The powerful optogenetic pharmacology method allows the optical control of neuronal activity by photoswitchable ligands tethered to channels and receptors. However, this approach is technically demanding, as it requires the design of pharmacologically active ligands. The development of versatile technologies therefore represents a challenging issue. Here, we present optogating, a method in which the gating machinery of an ATP-activated P2X channel was reprogrammed to respond to light. We found that channels covalently modified by azobenzene-containing reagents at the transmembrane segments could be reversibly turned on and off by light, without the need of ATP, thus revealing an agonist-independent, light-induced gating mechanism. We demonstrate photocontrol of neuronal activity by a light-gated, ATP-insensitive P2X receptor, providing an original tool devoid of endogenous sensitivity to delineate P2X signaling in normal and pathological states. These findings open new avenues to specifically activate other ion channels independently of their natural stimulus.
Keywords: azobenzene photoswitch; purinergic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

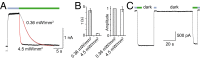
Similar articles
-
Optical control of trimeric P2X receptors and acid-sensing ion channels.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):521-6. doi: 10.1073/pnas.1318582111. Epub 2013 Dec 23. Proc Natl Acad Sci U S A. 2014. PMID: 24367083 Free PMC article.
-
Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):E3455-63. doi: 10.1073/pnas.1308088110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959888 Free PMC article.
-
Controlling Engineered P2X Receptors with Light.Methods Mol Biol. 2020;2041:301-309. doi: 10.1007/978-1-4939-9717-6_22. Methods Mol Biol. 2020. PMID: 31646498
-
Moving through the gate in ATP-activated P2X receptors.Trends Biochem Sci. 2013 Jan;38(1):20-9. doi: 10.1016/j.tibs.2012.10.006. Epub 2012 Dec 1. Trends Biochem Sci. 2013. PMID: 23206935 Review.
-
P2X Receptor Activation.Adv Exp Med Biol. 2017;1051:55-69. doi: 10.1007/5584_2017_55. Adv Exp Med Biol. 2017. PMID: 28639248 Review.
Cited by
-
Optical control of PIEZO1 channels.Nat Commun. 2023 Mar 7;14(1):1269. doi: 10.1038/s41467-023-36931-0. Nat Commun. 2023. PMID: 36882406 Free PMC article.
-
Key sites for P2X receptor function and multimerization: overview of mutagenesis studies on a structural basis.Curr Med Chem. 2015;22(7):799-818. doi: 10.2174/0929867322666141128163215. Curr Med Chem. 2015. PMID: 25439586 Free PMC article. Review.
-
A fine-tuned azobenzene for enhanced photopharmacology in vivo.Cell Chem Biol. 2021 Nov 18;28(11):1648-1663.e16. doi: 10.1016/j.chembiol.2021.02.020. Epub 2021 Mar 17. Cell Chem Biol. 2021. PMID: 33735619 Free PMC article.
-
Intersubunit physical couplings fostered by the left flipper domain facilitate channel opening of P2X4 receptors.J Biol Chem. 2017 May 5;292(18):7619-7635. doi: 10.1074/jbc.M116.771121. Epub 2017 Mar 16. J Biol Chem. 2017. PMID: 28302727 Free PMC article.
-
Light-Switchable Ion Channels and Receptors for Optogenetic Interrogation of Neuronal Signaling.Bioconjug Chem. 2018 Apr 18;29(4):861-869. doi: 10.1021/acs.bioconjchem.7b00803. Epub 2018 Feb 21. Bioconjug Chem. 2018. PMID: 29465988 Free PMC article. Review.
References
-
- Fehrentz T, Schönberger M, Trauner D. Optochemical genetics. Angew Chem Int Ed Engl. 2011;50(51):12156–12182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

