Stoichiometry for activation of neuronal α7 nicotinic receptors
- PMID: 24297903
- PMCID: PMC3870720
- DOI: 10.1073/pnas.1315775110
Stoichiometry for activation of neuronal α7 nicotinic receptors
Abstract
Neuronal α7 nicotinic receptors elicit rapid cation influx in response to acetylcholine (ACh) or its hydrolysis product choline. They contribute to cognition, synaptic plasticity, and neuroprotection and have been implicated in neurodegenerative and neuropsychiatric disorders. α7, however, often localizes distal to sites of nerve-released ACh and binds ACh with low affinity, and thus elicits its biological response with low agonist occupancy. To assess the function of α7 when ACh occupies fewer than five of its identical binding sites, we measured the open-channel lifetime of individual receptors in which four of the five ACh binding sites were disabled. To improve the time resolution of the inherently brief α7 channel openings, background mutations or a potentiator was used to increase open duration. We find that, in receptors with only one intact binding site, the open-channel lifetime is indistinguishable from receptors with five intact binding sites, counter to expectations from prototypical neurotransmitter-gated ion channels where the open-channel lifetime increases with the number of binding sites occupied by agonist. Replacing the membrane-embedded domain of α7 by that of the related 5-HT3A receptor increases the number of sites that need to be occupied to achieve the maximal open-channel lifetime, thus revealing a unique interdependence between the detector and actuator domains of these receptors. The distinctive ability of a single occupancy to elicit a full biological response adapts α7 to volume transmission, a prevalent mechanism of ACh-mediated signaling in the nervous system and nonneuronal cells.
Keywords: Cys-loop receptors; agonist binding site; channel gating; patch-clamp; α7 nicotinic receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
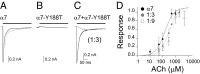
 ) and 1:9 subunit ratios (○)]. Each point corresponds to the mean ± SD.
) and 1:9 subunit ratios (○)]. Each point corresponds to the mean ± SD.



References
-
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. - PubMed
-
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. - PubMed
-
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: From animal models to human pathophysiology. Curr Pharm Des. 2010;16(3):323–343. - PubMed
-
- Wallace TL, Porter RH. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharmacol. 2011;82(8):891–903. - PubMed
-
- Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol Ther. 2013;137(1):22–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

