Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo
- PMID: 24297994
- PMCID: PMC3865483
- DOI: 10.1084/jem.20130733
Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo
Abstract
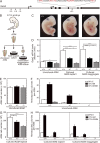
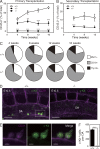
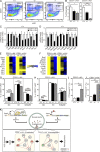
The generation of hematopoietic stem cells (HSCs) from hemogenic endothelium within the aorta, gonad, mesonephros (AGM) region of the mammalian embryo is crucial for development of the adult hematopoietic system. We described a deletion of a Gata2 cis-element (+9.5) that depletes fetal liver HSCs, is lethal at E13-14 of embryogenesis, and is mutated in an immunodeficiency that progresses to myelodysplasia/leukemia. Here, we demonstrate that the +9.5 element enhances Gata2 expression and is required to generate long-term repopulating HSCs in the AGM. Deletion of the +9.5 element abrogated the capacity of hemogenic endothelium to generate HSC-containing clusters in the aorta. Genomic analyses indicated that the +9.5 element regulated a rich ensemble of genes that control hemogenic endothelium and HSCs, as well as genes not implicated in hematopoiesis. These results reveal a mechanism that controls stem cell emergence from hemogenic endothelium to establish the adult hematopoietic system.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- DK68634/DK/NIDDK NIH HHS/United States
- R01 HL113066/HL/NHLBI NIH HHS/United States
- R01 HG003747/HG/NHGRI NIH HHS/United States
- HG005232/HG/NHGRI NIH HHS/United States
- HL113066/HL/NHLBI NIH HHS/United States
- DK50107/DK/NIDDK NIH HHS/United States
- CA152108/CA/NCI NIH HHS/United States
- R37 DK050107/DK/NIDDK NIH HHS/United States
- R01 DK050107/DK/NIDDK NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- R01 DK068634/DK/NIDDK NIH HHS/United States
- U01 HG007019/HG/NHGRI NIH HHS/United States
- R01 HG005232/HG/NHGRI NIH HHS/United States
- HG007019/HG/NHGRI NIH HHS/United States
- R56 DK068634/DK/NIDDK NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

